当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
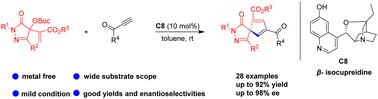
Asymmetric [3+2] spiroannulation of pyrazolone-derived Morita–Baylis–Hillman carbonates with alkynyl ketones: facile access to spiro[cyclopentadiene-pyrazolone] scaffolds
Chemical Communications ( IF 4.9 ) Pub Date : 2022-07-28 , DOI: 10.1039/d2cc02963d Xingfu Wei 1 , Yue Huang 1 , Wenyao Wang 1 , Shiqiang Wei 1 , Jingping Qu 1 , Baomin Wang 1
Chemical Communications ( IF 4.9 ) Pub Date : 2022-07-28 , DOI: 10.1039/d2cc02963d Xingfu Wei 1 , Yue Huang 1 , Wenyao Wang 1 , Shiqiang Wei 1 , Jingping Qu 1 , Baomin Wang 1
Affiliation
|
A tertiary amine-catalyzed asymmetric [3+2] spiroannulation reaction of pyrazolone-derived Morita–Baylis–Hillman carbonates with alkynyl ketones was achieved under mild conditions. This protocol offers a facile approach to chiral spiro[cyclopentadiene-pyrazolone] scaffolds in good to high yields (up to 92%) with good degrees of enantiocontrol (up to 98% ee). In addition, scale-up reaction and transformation of the products were performed to show the synthetic utility of this protocol.
中文翻译:

吡唑啉酮衍生的 Morita-Baylis-Hillman 碳酸盐与炔基酮的不对称 [3+2] 螺环化:容易获得螺[环戊二烯-吡唑啉酮] 支架
在温和条件下实现了吡唑啉酮衍生的 Morita-Baylis-Hillman 碳酸盐与炔基酮的叔胺催化不对称 [3+2] 螺环化反应。该协议为手性螺[环戊二烯-吡唑啉酮] 支架提供了一种简便的方法,具有良好的对映控制度(高达 98% ee)的良好至高产率(高达 92%)。此外,还对产品进行了放大反应和转化,以展示该协议的合成效用。
更新日期:2022-07-28
中文翻译:

吡唑啉酮衍生的 Morita-Baylis-Hillman 碳酸盐与炔基酮的不对称 [3+2] 螺环化:容易获得螺[环戊二烯-吡唑啉酮] 支架
在温和条件下实现了吡唑啉酮衍生的 Morita-Baylis-Hillman 碳酸盐与炔基酮的叔胺催化不对称 [3+2] 螺环化反应。该协议为手性螺[环戊二烯-吡唑啉酮] 支架提供了一种简便的方法,具有良好的对映控制度(高达 98% ee)的良好至高产率(高达 92%)。此外,还对产品进行了放大反应和转化,以展示该协议的合成效用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号