Journal of Controlled Release ( IF 10.8 ) Pub Date : 2022-07-27 , DOI: 10.1016/j.jconrel.2022.07.026 Rinie Bajracharya 1 , Esteban Cruz 1 , Jürgen Götz 1 , Rebecca M Nisbet 2
|
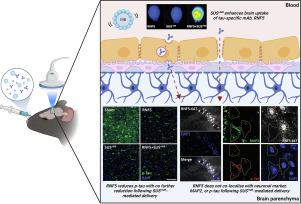
Tau-specific immunotherapy is an attractive strategy for the treatment of Alzheimer's disease and other tauopathies. However, effectively targeting tau in the brain remains a considerable challenge due to the restrictive nature of the blood-brain barrier (BBB), which excludes an estimated >99% of peripherally administered antibodies. However, their transport across the BBB can be facilitated by a novel modality, low-intensity scanning ultrasound used in combination with intravenously injected microbubbles (SUS+MB). We have previously shown that SUS+MB-mediated delivery of a tau-specific antibody in a single-chain (scFv) format to tau transgenic mice enhanced brain and neuronal uptake and subsequently, reduced tau pathology and improved behavioural outcomes to a larger extent than either scFv or SUS+MB on its own. Here we generated a novel tau-specific monoclonal antibody, RNF5, and validated it in its IgG format in the presence or absence of SUS+MB by treating K369I tau transgenic K3 mice once weekly for 12 weeks. We found that both RNF5 and SUS+MB treatments on their own significantly reduced tau pathology. In the combination group (RNF5 + SUS+MB), however, despite increased antibody localization in the brain, there were no further reductions in tau pathology when compared to RNF5 treatment alone. Furthermore, following SUS+MB, RNF5 accumulated heavily within cells across the pyramidal cell layer of the hippocampus, that were negative for MAP2 and p-tau, suggesting that SUS+MB may not facilitate enhanced RNF5 engagement of intraneuronal tau. Overall, our new findings reveal the complexities of combining tau immunotherapy with SUS+MB and challenge the view that this is a straight-forward approach.
中文翻译:

超声介导的新型 tau 特异性单克隆抗体的递送增强了脑摄取,但不增强治疗效果
Tau 特异性免疫疗法是治疗阿尔茨海默病和其他 tau 病变的有吸引力的策略。然而,由于血脑屏障 (BBB) 的限制性性质,有效靶向大脑中的 tau 仍然是一个相当大的挑战,它排除了估计 >99% 的外周给药抗体。然而,它们通过 BBB 的运输可以通过与静脉注射微泡 (SUS +MB )结合使用的新型低强度扫描超声来促进。我们之前已经证明 SUS +MB以单链 (scFv) 形式向 tau 转基因小鼠介导的 tau 特异性抗体的递送增强了大脑和神经元的摄取,随后,与 scFv 或 SUS +MB相比,在更大程度上减少了 tau 病理学并改善了行为结果自己的。在这里,我们生成了一种新的 tau 特异性单克隆抗体 RNF5,并通过每周一次治疗 K369I tau 转基因 K3 小鼠持续 12 周,在存在或不存在 SUS + MB的情况下验证其 IgG 形式。我们发现 RNF5 和 SUS + MB治疗本身都显着降低了 tau 病理学。在组合组(RNF5 + SUS +MB),然而,尽管抗体在大脑中的定位增加,与单独的 RNF5 治疗相比,tau 病理学没有进一步减少。此外,在 SUS +MB之后,RNF5 在海马锥体细胞层的细胞内大量积累,对 MAP2 和 p-tau 呈阴性,这表明 SUS +MB可能不会促进增强的 RNF5 参与神经元内 tau。总体而言,我们的新发现揭示了将 tau 免疫疗法与 SUS + MB相结合的复杂性,并挑战了认为这是一种直截了当的方法的观点。


























 京公网安备 11010802027423号
京公网安备 11010802027423号