Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
In Situ Imaging of Endogenous Hydrogen Peroxide Efflux from Living Cells via Bipolar Gold Nanoelectrode Array and Electrochemiluminescence Technology
ACS Sensors ( IF 8.9 ) Pub Date : 2022-07-25 , DOI: 10.1021/acssensors.2c01195 Xiuxiu Li 1 , Xiang Qin 2 , Zhi Wang 3 , Yafeng Wu 1 , Kang Wang 2 , Xinghua Xia 2 , Songqin Liu 1
ACS Sensors ( IF 8.9 ) Pub Date : 2022-07-25 , DOI: 10.1021/acssensors.2c01195 Xiuxiu Li 1 , Xiang Qin 2 , Zhi Wang 3 , Yafeng Wu 1 , Kang Wang 2 , Xinghua Xia 2 , Songqin Liu 1
Affiliation
|
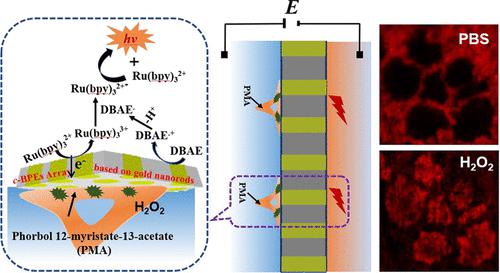
The integration of a closed bipolar electrode (c-BPE) array and electrochemiluminescence (ECL) detection received a boost in applications in the detection of cell adhesion and disease-related biomarkers. This work proposed a gold nanorod array based c-BPE–ECL system to realize an in situ image of endogenous hydrogen peroxide (H2O2) efflux from living cells and parallel analysis of endogenous H2O2 released from multiple cells by converting electrochemical signals into optical signals. The gold nanorod array with high density was prepared by a repeating chronopotentiometry procedure with anodic aluminum oxide (AAO) membrane as a template. The c-BPE array was fabricated by assembling poly(dimethylsiloxane) (PDMS) chips on both sides of the gold nanorod array. When an appropriate driving potential is applied, H2O2 generated from living cells at the sensing pole was reduced on the gold nanorod, triggering the oxidation of the ECL reagent at the reporting pole, which allowed the detection of H2O2 released from living cells. Under phorbol myristate acetate (PMA) stimulation, H2O2 released from living HeLa, HepG2, MCF-7, and LO2 cells was determined to be 47, 32.4, 25.7, and 6.3 μM, respectively. This indicated that the amount of H2O2 released from PMA-stimulated cancer cells was significantly higher than that from the stimulated normal cells. This work presented a new approach for in situ imaging of H2O2 released from living cells and could also be used to detect other electrochemically active or non-electrochemically active molecules through simple cell surface modification, which may have potential applications in cell apoptosis study and disease diagnosis.
中文翻译:

通过双极金纳米电极阵列和电化学发光技术对活细胞内源性过氧化氢流出进行原位成像
封闭双极电极 ( c -BPE) 阵列和电化学发光 (ECL) 检测的集成在检测细胞粘附和疾病相关生物标志物的应用中得到了推动。这项工作提出了一种基于金纳米棒阵列的c -BPE-ECL 系统,以实现活细胞内源性过氧化氢 (H 2 O 2 ) 流出的原位成像,以及通过电化学转换对多个细胞释放的内源性 H 2 O 2进行并行分析信号转换成光信号。以阳极氧化铝 (AAO) 膜为模板,通过重复计时电位法制备高密度金纳米棒阵列。c _-BPE 阵列是通过在金纳米棒阵列的两侧组装聚二甲基硅氧烷 (PDMS) 芯片制成的。当施加适当的驱动电位时,传感极活细胞产生的H 2 O 2在金纳米棒上被还原,触发报告极ECL试剂的氧化,从而检测释放的H 2 O 2活细胞。在醋酸佛波醇肉豆蔻酸酯 (PMA) 刺激下,活体 HeLa、HepG2、MCF-7 和 LO2 细胞释放的 H 2 O 2分别为 47、32.4、25.7 和 6.3 μM。这表明 H 2 O 2的量PMA 刺激的癌细胞释放的 PMA 显着高于刺激的正常细胞。该工作为活细胞释放的H 2 O 2原位成像提供了一种新方法,也可用于通过简单的细胞表面修饰检测其他电化学活性或非电化学活性分子,这可能在细胞凋亡研究中具有潜在应用和疾病诊断。
更新日期:2022-07-25
中文翻译:

通过双极金纳米电极阵列和电化学发光技术对活细胞内源性过氧化氢流出进行原位成像
封闭双极电极 ( c -BPE) 阵列和电化学发光 (ECL) 检测的集成在检测细胞粘附和疾病相关生物标志物的应用中得到了推动。这项工作提出了一种基于金纳米棒阵列的c -BPE-ECL 系统,以实现活细胞内源性过氧化氢 (H 2 O 2 ) 流出的原位成像,以及通过电化学转换对多个细胞释放的内源性 H 2 O 2进行并行分析信号转换成光信号。以阳极氧化铝 (AAO) 膜为模板,通过重复计时电位法制备高密度金纳米棒阵列。c _-BPE 阵列是通过在金纳米棒阵列的两侧组装聚二甲基硅氧烷 (PDMS) 芯片制成的。当施加适当的驱动电位时,传感极活细胞产生的H 2 O 2在金纳米棒上被还原,触发报告极ECL试剂的氧化,从而检测释放的H 2 O 2活细胞。在醋酸佛波醇肉豆蔻酸酯 (PMA) 刺激下,活体 HeLa、HepG2、MCF-7 和 LO2 细胞释放的 H 2 O 2分别为 47、32.4、25.7 和 6.3 μM。这表明 H 2 O 2的量PMA 刺激的癌细胞释放的 PMA 显着高于刺激的正常细胞。该工作为活细胞释放的H 2 O 2原位成像提供了一种新方法,也可用于通过简单的细胞表面修饰检测其他电化学活性或非电化学活性分子,这可能在细胞凋亡研究中具有潜在应用和疾病诊断。



























 京公网安备 11010802027423号
京公网安备 11010802027423号