Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Adipose microtissue-on-chip: a 3D cell culture platform for differentiation, stimulation, and proteomic analysis of human adipocytes
Lab on a Chip ( IF 6.1 ) Pub Date : 2022-07-25 , DOI: 10.1039/d2lc00245k Nina Compera 1 , Scott Atwell 1 , Johannes Wirth 1 , Christine von Törne 2 , Stefanie M Hauck 2 , Matthias Meier 1, 3
Lab on a Chip ( IF 6.1 ) Pub Date : 2022-07-25 , DOI: 10.1039/d2lc00245k Nina Compera 1 , Scott Atwell 1 , Johannes Wirth 1 , Christine von Törne 2 , Stefanie M Hauck 2 , Matthias Meier 1, 3
Affiliation
|
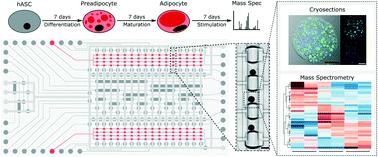
Human fat tissue has evolved to serve as a major energy reserve. An imbalance between energy intake and expenditure leads to an expansion of adipose tissue. Maintenance of this energy imbalance over long periods leads to obesity and metabolic disorders such as type 2 diabetes, for which a clinical cure is not yet available. In this study, we developed a microfluidic large-scale integration chip platform to automate the formation, long-term culture, and retrieval of 3D adipose microtissues to enable longitudinal studies of adipose tissue in vitro. The chip was produced from soft-lithography molds generated by 3D-printing, which allowed scaling of pneumatic membrane valves for parallel fluid routing and thus incorporated microchannels with variable dimensions to handle 3D cell cultures with diameters of several hundred micrometers. In 32 individual fluidically accessible cell culture chambers, designed to enable the self-aggregation process of three microtissues, human adipose stem cells differentiated into mature adipocytes over a period of two weeks. Coupling mass spectrometry to the cell culture platform, we determined the minimum cell numbers required to obtain robust and complex proteomes with over 1800 identified proteins. The adipose microtissues on the chip platform were then used to periodically simulate food intake by alternating the glucose level in the cell-feeding media every 6 h over the course of one week. The proteomes of adipocytes under low/high glucose conditions exhibited unique protein profiles, confirming the technical functionality and applicability of the chip platform. Thus, our adipose tissue-on-chip in vitro model may prove useful for elucidating the molecular and functional mechanisms of adipose tissue in normal and pathological conditions, such as obesity.
中文翻译:

芯片上脂肪微组织:用于人类脂肪细胞分化、刺激和蛋白质组分析的 3D 细胞培养平台
人类脂肪组织已经发展成为主要的能量储备。能量摄入和消耗之间的不平衡导致脂肪组织扩张。长期维持这种能量失衡会导致肥胖和代谢紊乱,例如 2 型糖尿病,而目前尚无临床治愈方法。在这项研究中,我们开发了一种微流控大规模集成芯片平台,可以自动化3D脂肪微组织的形成、长期培养和检索,从而实现体外脂肪组织的纵向研究。该芯片由 3D 打印生成的软光刻模具制成,允许缩放气动膜阀以实现并行流体路由,从而合并具有可变尺寸的微通道,以处理直径为数百微米的 3D 细胞培养物。在 32 个独立的可流体访问的细胞培养室中,人类脂肪干细胞在两周内分化为成熟脂肪细胞,这些细胞培养室旨在实现三个微组织的自我聚集过程。将质谱法与细胞培养平台相结合,我们确定了获得具有 1800 多种已鉴定蛋白质的稳健且复杂的蛋白质组所需的最小细胞数。然后,芯片平台上的脂肪微组织被用来定期模拟食物摄入,方法是在一周的时间内每 6 小时改变细胞喂养培养基中的葡萄糖水平。低/高葡萄糖条件下脂肪细胞的蛋白质组表现出独特的蛋白质谱,证实了该芯片平台的技术功能和适用性。因此,我们的脂肪组织芯片体外模型可能有助于阐明正常和病理条件下(例如肥胖)脂肪组织的分子和功能机制。
更新日期:2022-07-25
中文翻译:

芯片上脂肪微组织:用于人类脂肪细胞分化、刺激和蛋白质组分析的 3D 细胞培养平台
人类脂肪组织已经发展成为主要的能量储备。能量摄入和消耗之间的不平衡导致脂肪组织扩张。长期维持这种能量失衡会导致肥胖和代谢紊乱,例如 2 型糖尿病,而目前尚无临床治愈方法。在这项研究中,我们开发了一种微流控大规模集成芯片平台,可以自动化3D脂肪微组织的形成、长期培养和检索,从而实现体外脂肪组织的纵向研究。该芯片由 3D 打印生成的软光刻模具制成,允许缩放气动膜阀以实现并行流体路由,从而合并具有可变尺寸的微通道,以处理直径为数百微米的 3D 细胞培养物。在 32 个独立的可流体访问的细胞培养室中,人类脂肪干细胞在两周内分化为成熟脂肪细胞,这些细胞培养室旨在实现三个微组织的自我聚集过程。将质谱法与细胞培养平台相结合,我们确定了获得具有 1800 多种已鉴定蛋白质的稳健且复杂的蛋白质组所需的最小细胞数。然后,芯片平台上的脂肪微组织被用来定期模拟食物摄入,方法是在一周的时间内每 6 小时改变细胞喂养培养基中的葡萄糖水平。低/高葡萄糖条件下脂肪细胞的蛋白质组表现出独特的蛋白质谱,证实了该芯片平台的技术功能和适用性。因此,我们的脂肪组织芯片体外模型可能有助于阐明正常和病理条件下(例如肥胖)脂肪组织的分子和功能机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号