Redox Biology ( IF 11.4 ) Pub Date : 2022-07-22 , DOI: 10.1016/j.redox.2022.102418 Ping Guo 1 , Shijia Zu 2 , Shilong Han 1 , Wendan Yu 1 , Guoqing Xue 1 , Xiaona Lu 1 , Hua Lin 3 , Xinrui Zhao 1 , Haibo Lu 4 , Chunyu Hua 1 , Xinyu Wan 1 , Liyuan Ru 1 , Ziyue Guo 1 , Hanxiao Ge 1 , Kuan Lv 1 , Guohui Zhang 1 , Wuguo Deng 5 , Cheng Luo 6 , Wei Guo 1
|
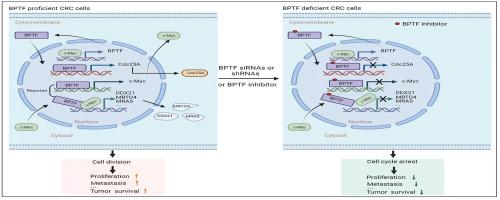
As the largest subunit of the nuclear remodeling factor complex, Bromodomain PHD Finger Transcription Factor (BPTF) has been reported to be involved in tumorigenesis and development in several cancers. However, to date, its functions and related molecular mechanisms in colorectal cancer (CRC) are still poorly defined and deserve to be revealed. In this study, we uncovered that, under the expression regulation of c-Myc, BPTF promoted CRC progression by targeting Cdc25A. BPTF was found to be highly expressed in CRC and promoted the proliferation and metastasis of CRC cells through BPTF specific siRNAs, shRNAs or inhibitors. Based on RNA-seq, combined with DNA-pulldown, ChIP and luciferase reporter assay, we proved that, by binding to −178/+107 region within Cdc25A promoter, BPTF transcriptionally activated Cdc25A, thus accelerating the cell cycle process of CRC cells. Meanwhile, BPTF itself was found to be transcriptionally regulated by c-Myc. Moreover, BPTF knockdown or inactivation was verified to sensitize CRC cells to chemotherapeutics, 5-Fluorouracil (5FU) and Oxaliplatin (Oxa), c-Myc inhibitor and cell cycle inhibitor not just at the cellular level in vitro, but in subcutaneous xenografts or AOM/DSS-induced in situ models of CRC in mice, while Cdc25A overexpression partially reversed BPTF silencing-caused tumor growth inhibition. Clinically, BPTF, c-Myc and Cdc25A were highly expressed in CRC tissues simultaneously, the expression of any two of the three was positively correlated, and their expressions were highly relevant to tumor differentiation, TNM staging and poor prognosis of CRC patients. Thus, our study indicated that the targeted inhibition of BPTF alone, or together with chemotherapy and/or cell cycle-targeted therapy, might act as a promising new strategy for CRC treatment, while c-Myc/BPTF/Cdc25A signaling axis is expected to be developed as an associated set of candidate biomarkers for CRC diagnosis and prognosis prediction.
中文翻译:

BPTF 抑制通过转录灭活 Cdc25A 来拮抗结直肠癌进展
作为核重塑因子复合物的最大亚基,Bromodomain PHD Finger 转录因子 (BPTF) 据报道参与多种癌症的肿瘤发生和发展。然而,迄今为止,其在结直肠癌(CRC)中的功能和相关分子机制仍不清楚,值得揭示。在本研究中,我们发现,在c-Myc的表达调控下,BPTF通过靶向Cdc25A促进CRC进展。研究发现BPTF在CRC中高表达,并通过BPTF特异性siRNA、shRNA或抑制剂促进CRC细胞的增殖和转移。基于RNA-seq,结合DNA-pulldown、ChIP和荧光素酶报告基因检测,我们证明,BPTF通过与Cdc25A启动子内的-178/+107区域结合,转录激活Cdc25A,从而加速CRC细胞的细胞周期过程。同时,BPTF本身也被发现受到c-Myc的转录调控。此外,BPTF 敲低或失活已被证实不仅在体外细胞水平上,而且在皮下异种移植物或 AOM 中都可以使 CRC 细胞对化疗药物、5-氟尿嘧啶 (5FU) 和奥沙利铂 (Oxa)、c-Myc 抑制剂和细胞周期抑制剂敏感。 /DSS 诱导的小鼠 CRC 原位模型,而 Cdc25A 过表达部分逆转了 BPTF 沉默引起的肿瘤生长抑制。临床上,BPTF、c-Myc和Cdc25A在CRC组织中同时高表达,三者中任意两者表达呈正相关,其表达与CRC患者的肿瘤分化、TNM分期及不良预后高度相关。因此,我们的研究表明,单独靶向抑制 BPTF,或与化疗和/或细胞周期靶向治疗联合使用,可能成为 CRC 治疗的一种有前景的新策略,而 c-Myc/BPTF/Cdc25A 信号轴有望被开发为一组相关的候选生物标志物,用于 CRC 诊断和预后预测。



























 京公网安备 11010802027423号
京公网安备 11010802027423号