当前位置:
X-MOL 学术
›
Biotechnol. Bioeng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enhancing thermal stability and lytic activity of phage lysin PlyAB1 from Acinetobacter baumannii
Biotechnology and Bioengineering ( IF 3.8 ) Pub Date : 2022-07-20 , DOI: 10.1002/bit.28187 Yingbo Yuan 1 , Qingbin Li 1 , Shuhang Zhang 1 , Jinhong Gu 1 , Guangtao Huang 2 , Qingsheng Qi 1 , Xuemei Lu 1
Biotechnology and Bioengineering ( IF 3.8 ) Pub Date : 2022-07-20 , DOI: 10.1002/bit.28187 Yingbo Yuan 1 , Qingbin Li 1 , Shuhang Zhang 1 , Jinhong Gu 1 , Guangtao Huang 2 , Qingsheng Qi 1 , Xuemei Lu 1
Affiliation
|
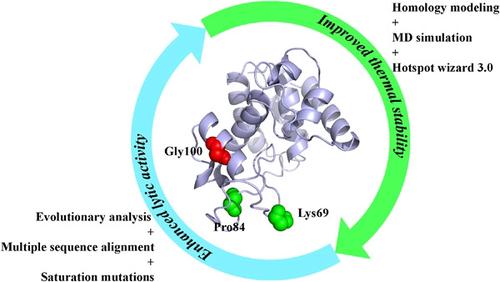
With the increasingly serious drug resistance of Acinetobacter baumannii, there is an increasingly urgent need for new antibacterial drugs. Phage lysin PlyAB1 has a bactericidal effect on drug-resistant A. baumannii, which has the potential to replace antibiotics to fight infection caused by A. baumannii. However, its application is limited by its thermal stability and lytic activity. To solve these problems, molecular dynamics (MD) simulations combined with Hotspot wizard 3.0 were used to identify key residue sites affecting thermal stability, and evolutionary analysis combined with multiple sequence alignment was used to identify key residue sites affecting lytic activity. Four single-point variants with significantly increased thermal stability and four single-point variants with significantly lytic activity were obtained, respectively. Furthermore, by superimposing mutations, we obtained three double-point variants, G100Q/K69R, G100R/K69R, and G100K/K69R, with significantly improved thermal stability and improved lytic activity. At 45°C, the lytic activity and half-life of the optimal variant G100Q/K69R were 1.51- and 24-fold higher than those of the wild PlyAB1, respectively. These results deepen our understanding of the structure and function of phage lysin and contribute to the application of phage lysin in antibiotic substitution.
中文翻译:

增强鲍曼不动杆菌噬菌体溶素 PlyAB1 的热稳定性和裂解活性
随着鲍曼不动杆菌耐药性的日益严重,对新型抗菌药物的需求日益迫切。噬菌体溶素PlyAB1对耐药鲍曼不动杆菌具有杀菌作用,有望替代抗生素对抗鲍曼不动杆菌引起的感染. 然而,它的应用受到其热稳定性和裂解活性的限制。为了解决这些问题,分子动力学 (MD) 模拟结合 Hotspot Wizard 3.0 被用来识别影响热稳定性的关键残基位点,进化分析结合多序列比对被用来识别影响裂解活性的关键残基位点。分别获得了四个热稳定性显着提高的单点变体和四个具有显着裂解活性的单点变体。此外,通过叠加突变,我们获得了三个双点变体,G100Q/K69R、G100R/K69R 和 G100K/K69R,具有显着提高的热稳定性和裂解活性。在 45°C 时,最佳变体 G100Q/K69R 的裂解活性和半衰期为 1。分别比野生 PlyAB1 高 51 倍和 24 倍。这些结果加深了我们对噬菌体溶素结构和功能的理解,有助于噬菌体溶素在抗生素替代中的应用。
更新日期:2022-07-20
中文翻译:

增强鲍曼不动杆菌噬菌体溶素 PlyAB1 的热稳定性和裂解活性
随着鲍曼不动杆菌耐药性的日益严重,对新型抗菌药物的需求日益迫切。噬菌体溶素PlyAB1对耐药鲍曼不动杆菌具有杀菌作用,有望替代抗生素对抗鲍曼不动杆菌引起的感染. 然而,它的应用受到其热稳定性和裂解活性的限制。为了解决这些问题,分子动力学 (MD) 模拟结合 Hotspot Wizard 3.0 被用来识别影响热稳定性的关键残基位点,进化分析结合多序列比对被用来识别影响裂解活性的关键残基位点。分别获得了四个热稳定性显着提高的单点变体和四个具有显着裂解活性的单点变体。此外,通过叠加突变,我们获得了三个双点变体,G100Q/K69R、G100R/K69R 和 G100K/K69R,具有显着提高的热稳定性和裂解活性。在 45°C 时,最佳变体 G100Q/K69R 的裂解活性和半衰期为 1。分别比野生 PlyAB1 高 51 倍和 24 倍。这些结果加深了我们对噬菌体溶素结构和功能的理解,有助于噬菌体溶素在抗生素替代中的应用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号