Functional Ecology ( IF 5.2 ) Pub Date : 2022-07-19 , DOI: 10.1111/1365-2435.14147 Rory S. Telemeco 1 , Eric J. Gangloff 2 , G. Antonio Cordero 3 , Essie M. Rodgers 4 , Fabien Aubret 5
|
1 INTRODUCTION
Abiotic factors, such as temperature and oxygen availability, influence the vital rates of organisms both directly and through their interaction (Angilletta, 2009; Ern, 2019; Gangloff & Telemeco, 2018; Rezende et al., 2014). Understanding the relationship between abiotic factors and organismal performance has long been an aim of biologists, with the global climate and biodiversity crises adding urgency to this goal. Perhaps the best-developed paradigm describing the relationship between organismal performance and an abiotic factor is the thermal performance curve (TPC; Angilletta, 2009; Huey & Stevenson, 1979; Taylor et al., 2021). Primarily applicable to ectothermic organisms, TPCs quantify the performance or functionality of a physiological process or integrated performance measure across the range of temperatures at which these processes can occur. Importantly, such curves are applicable across levels of biological organization, from subcellular processes (Licht, 1964; Somero, 2020) to metabolism (Clark et al., 2013; Schulte, 2015) to whole-organism performance (Gilbert & Miles, 2019; Stevenson et al., 1985) to population growth (Luhring & DeLong, 2016; Ratkowsky et al., 2005). From these curves, parameters describing organismal function can be extracted, including the maximal performance, optimal temperature for performance, upper and lower thermal limits, and performance breadth (Angilletta, 2009; Taylor et al., 2021). These parameters, as well as their variation across traits, individuals, and populations, are used in myriad applications, including modelling responses to novel or changing environments (Deutsch et al., 2008; Kearney et al., 2008; Levy et al., 2015).
While undoubtedly useful, TPCs have limitations. For example, TPCs are descriptive representations of the thermal dependence of traits measured in a specific time and place (typically a controlled laboratory environment), and their context dependence makes them difficult to extrapolate to diverse natural conditions (Bodensteiner, Agudelo-Cantero, et al., 2021; Gangloff & Telemeco, 2018; Kingsolver & Buckley, 2020; Rezende et al., 2014; Schulte et al., 2011; Woods et al., 2018). Furthermore, TPCs are univariate models, despite physiological traits responding to numerous interacting abiotic factors in nature. In this study, we explore the link between aerobic capacity and thermal performance (Ern, 2019; Gangloff & Telemeco, 2018; Pörtner et al., 2017; Pörtner & Knust, 2007; Schulte, 2015). The oxygen- and capacity-limited thermal tolerance hypothesis (OCLTT) proposes that whole-organism thermal performance is constrained by an organism's metabolic demands and oxygen supply capacity, with organismal thermal limits set when oxygen demands at extreme temperatures exceed the organism's capacity to acquire and circulate oxygen (Pörtner, 2001; Pörtner, 2002). The OCLTT hypothesis has received mixed support in diverse taxa (Ern et al., 2016; Gangloff & Telemeco, 2018; Pörtner & Knust, 2007; Verberk et al., 2016) and induced heated debate about its ecological relevance and universality (Clark et al., 2013; Jutfelt et al., 2018; Pörtner et al., 2018).
More recently, the Hierarchical Mechanisms of Thermal Limitation (HMTL) hypothesis was developed to reconcile observations that support and refute OCLTT in ectothermic animals (Box 1; Gangloff & Telemeco, 2018). Similar to OCLTT, the HMTL hypothesis proposes that whole-organism performance is driven by aerobic capacity across suitable temperatures. However, HMTL explicitly recognizes that critical thermal limits can be set by multiple mechanisms, including lack of aerobic capacity and failure of subcellar components such as proteins or membranes. Whether limits on aerobic capacity or subcellular functionality are reached first proximally sets thermal limits, and this hierarchy can change predictably with life-history stage and environmental context. In addition to mechanistically explaining the asymmetrical shape of the TPC characteristic of many ectotherms, HMTL leads to specific predictions about how TPC shape is affected by oxygen environment and capacity (Box 1; for full details, see Gangloff & Telemeco, 2018). Principally, HMTL predicts that peak performance and the optimal temperature for performance are reduced when the organism's ability to obtain or utilize oxygen becomes limited, even when thermal limits, such as the critical thermal maximum (CTMAX), are unaffected. Invasion of high elevation, breath-holding, intense activity, pregnancy, disease and ontogenetic stage are common circumstances where reduced aerobic capacity could meaningfully affect TPC shape in ectotherms. Thus, the HMTL framework could provide an essential tool in predicting organismal responses to novel or changing environments. Such quantitative predictions about both the sublethal and lethal effects of extreme temperatures across life-history stages or environments that differ in oxygen capacity or availability are especially prescient in ecological contexts presented by the global climate emergency, including in the context of warming waters or upslope migration (Ern et al., 2016; Jacobsen, 2020; Rodgers et al., 2021; Storz, 2021).
BOX 1. Hierarchical mechanisms of thermal limitation
The Hierarchical Mechanisms of Thermal Limitation hypothesis (HMTL; Gangloff & Telemeco, 2018) combines ideas from the Oxygen-and Capacity-Limited Thermal Tolerance hypothesis (OCLTT; Pörtner, 2001, 2002; Pörtner et al., 2017), Marginal Stability hypothesis (Hochachka & Somero, 2002) and Thermal Performance Curve paradigm (TPC; Huey & Stevenson, 1979) to mechanistically describe how temperature and oxygen availability interact to affect whole-organism performance in ectothermic animals. Figure B1 provides a schematic of the HMTL hypothesis. As with OCLTT, HMTL proposes that whole-organism performance is proportional to aerobic scope, the difference between resting metabolic demand and maximum metabolic capacity. As temperature increases above the critical thermal minimum (CTMIN), aerobic scope increases before plateauing when maximum metabolic rate (MMR) equals the maximum aerobic capacity of the organism (horizontal dashed lines in the top panels of Figure B1). As temperature increases above the thermal optimum for performance (TOPT), MMR remains fixed but resting metabolic rate (RMR) continues to increase due to thermal effects on metabolic kinetics, resulting in reduced aerobic scope. This slow drop in aerobic performance will continue until RMR equals MMR and aerobic scope equals zero (Figure B1). We call this point the ‘aerobic critical temperature’ (aerobic TCRIT). By contrast, we call the temperature where subcellular components critically lose function, such as when membranes and enzymes become too flexible or unstable to properly function (Marginal Stability Hypothesis; Hochachka & Somero, 2002), the ‘subcellular TCRIT’. While the breakdown of subcellular processes can (and likely will) lead to aerobic capacity failure, this process is distinct from organismal performance limits being determined directly by aerobic capacity, as defined at aerobic TCRIT. HMTL proposes that CTMAX is driven by whichever TCRIT is lower (hence ‘hierarchical mechanisms’ in HMTL). Under HMTL, any change in aerobic capacity that reduces aerobic TCRIT could induce a shift from subcellular to aerobic mechanisms driving CTMAX. This applies even in species typically limited by subcellular TCRIT, as illustrated for the ‘Extremely reduced aerobic capacity’ condition in Figure B1c. The primary conceptual advance of HMTL is its explicit acknowledgement that either oxygen limitation or marginal stability can underlie lost performance at high temperatures. Furthermore, this mechanism can transition plastically and predictably.
HMTL makes multiple novel and testable predictions for the shape of the TPC when either aerobic or subcellular TCRIT proximally set high-temperature limits, and for how TPC shape will change in response to variation in oxygen environment or aerobic capacity. When aerobic TCRIT underlies CTMAX, HMTL predicts that the TPC will be symmetrical (Figure B1c). This symmetry results from aerobic scope decreasing with temperature above TOPT at the same rate that aerobic scope increased with temperature below TOPT. However, when subcellular TCRIT underlies CTMAX, HMTL predicts that the TPC will be left skewed, as is typical of most ectotherms (Figure B1a,b). Moreover, HMTL predicts that this skew will increase with the distance between the aerobic TCRIT and subcellular TCRIT. This asymmetry results from subcellular TCRIT inducing rapid performance losses at temperatures below those that would induce major aerobic limitation due to a mismatch between demand and supply capacity. HMTL provides a framework that can be used to make mechanistic predictions about both critical thermal limits and how TPC shape is affected by the oxygen environment across ectothermic taxa and ecological contexts.

A further challenge to utilizing TPCs as predictive tools is the selection of relevant traits as proxies for whole-organism performance or fitness. TPCs can vary among traits (Clark et al., 2013; Kellermann et al., 2019; Stevenson et al., 1985) and respond differently to the interaction of temperature with factors such as oxygen. Thus, if a performance measure is to be used as a fitness proxy, the trait and context in which it is measured must be carefully considered. Locomotor performance, specifically sprint speed in lizards, is an easily and widely measured trait relevant to multiple ecological contexts including predator escape, foraging and conflicts between conspecifics (Gilbert & Miles, 2017; Miles, 2004). However, there are important considerations when utilizing this measure as a fitness proxy (Irschick, 2003). For example, sprint speed over short distances is almost entirely anaerobically fuelled in lizards (Bennett & Licht, 1972; Gleeson, 1991). As a result, lizards can only maintain sprinting performance for seconds-to-minutes before they must rest and use aerobic respiration to regain homeostasis (Gleeson, 1982; Gleeson & Hancock, 2002). Any application of anaerobic TPCs to predict organismal responses to long-term changes in temperature, such as result from climate change, implicitly assumes that aerobic performance capacity, which powers the majority of functions such as digestion, growth and reproduction, is at least as high as anaerobic performance across temperatures. If this assumption is not met, then anaerobic traits such as sprint speed will be poor proxies for whole-organism performance and more-aerobic traits should be preferred. Recent work emphasizes the ecological relevance of aerobic scope as an integrated measure of whole-organism performance. Aerobic scope is the difference between resting metabolic demand and maximum metabolic capacity, which represents the capacity for animals to power aerobic processes (Gangloff & Telemeco, 2018; Pörtner et al., 2017; Schulte, 2015; Seibel & Deutsch, 2020). TPCs for aerobic scope are useful in bridging laboratory measurements of individual animals to broader biogeographical patterns and predictions (Bozinovic & Pörtner, 2015; Clark et al., 2013; Pörtner, 2021). While both sprint speed and aerobic scope exhibit temperature dependence, they differ in how they are fuelled and thus likely differ in how they will respond to variation in the oxygen environment. Because sprint speed is almost entirely fuelled by anaerobic respiration (Bennett & Licht, 1972), it is not expected to be limited by acute reductions in oxygen availability, whereas maximal aerobic capacity, and therefore aerobic scope, will be constrained (Storz, 2021).
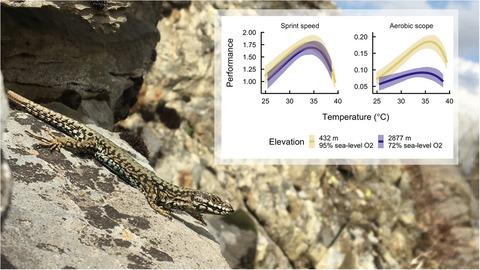
With this study, we test and support predictions of the HMTL hypothesis by measuring individual TPCs for both sprint speed and aerobic scope within the context of upslope-migrating common wall lizards Podarcis muralis. This widespread species was historically limited to elevations up to 2,000 m above sea level (ASL), but in recent years has been observed colonizing higher elevation habitat (routinely observed up to 2,300 m ASL; Pottier, 2020, F. Aubret, pers. obs.). Transplant experiments demonstrate that adult P. muralis lizards are oxygen limited at elevations above their current range limit, suffering from reduced aerobic scope and performance (Bodensteiner, Gangloff, et al., 2021; Gangloff et al., 2019). We hypothesize that TPCs for sprint speed and aerobic scope will exhibit broadly similar patterns when measured at low elevations, but that TPCs for aerobic scope will be affected by hypoxia at high elevation while sprint speed will remain unaffected. Specifically, as predicted by the HMTL hypothesis (Gangloff & Telemeco, 2018), we expect that both maximum aerobic scope and the optimal temperature for aerobic scope will be reduced at high elevation. These predictions arise because reduced oxygen availability causes the TPC for aerobic performance to become more symmetrical and flattened in shape, with maximum performance greatly reduced and the optimal temperature for peak performance reduced (Box 1, Figure B1b, Figure 1). Based on our empirical observations and HMTL, we then develop a mathematical framework for both describing and predicting the dual effects of temperature and environmental oxygen availability on organismal performance, Temperature-Oxygen Performance Surfaces (TOPS). Finally, we leverage this approach to make predictions about the potential for P. muralis to colonize high-elevation habitats.

中文翻译:

从性能曲线到性能表面:温度和氧气可用性对普通壁蜥好氧和厌氧性能的交互影响
1 简介
非生物因素,例如温度和氧气可用性,直接和通过它们的相互作用影响生物体的生命速率(Angilletta, 2009;Ern, 2019;Gangloff & Telemeco, 2018;Rezende 等, 2014)。长期以来,了解非生物因素与有机体性能之间的关系一直是生物学家的目标,全球气候和生物多样性危机增加了这一目标的紧迫性。描述有机体性能与非生物因素之间关系的最完善的范例可能是热性能曲线(TPC;Angilletta, 2009;Huey & Stevenson, 1979;Taylor 等人, 2021)。TPC 主要适用于变温生物,可量化生理过程的性能或功能,或在这些过程可能发生的温度范围内进行综合性能测量。重要的是,这些曲线适用于生物组织的各个层次,从亚细胞过程 (Licht, 1964 ; Somero, 2020 ) 到新陈代谢 (Clark et al., 2013 ; Schulte, 2015 ) 再到整个生物体的表现 (Gilbert & Miles, 2019 ; Stevenson et al., 1985 ) 到人口增长 (Luhring & DeLong, 2016 ; Ratkowsky et al., 2005)。从这些曲线中,可以提取描述有机体功能的参数,包括最大性能、性能的最佳温度、热上限和下限以及性能广度(Angilletta, 2009 年;Taylor 等人, 2021 年)。这些参数以及它们在性状、个体和群体之间的变化被用于无数应用中,包括对新的或不断变化的环境的响应建模(Deutsch 等人, 2008 年;Kearney 等人, 2008 年;Levy 等人, 2015 年)。
虽然无疑有用,但 TPC 也有局限性。例如,TPC 是在特定时间和地点(通常是受控的实验室环境)测量的性状的热依赖性的描述性表示,它们的上下文依赖性使得它们难以外推到不同的自然条件(Bodensteiner、Agudelo-Cantero 等人) ., 2021 ; Gangloff & Telemeco, 2018 ; Kingsolver & Buckley, 2020 ; Rezende 等人, 2014 ; Schulte 等人, 2011 ; Woods 等人, 2018)。此外,尽管生理特征对自然界中许多相互作用的非生物因素作出反应,但 TPC 是单变量模型。在这项研究中,我们探讨了有氧能力和热性能之间的联系(Ern, 2019 年;Gangloff 和 Telemeco, 2018 年;Pörtner 等人, 2017 年;Pörtner 和 Knust, 2007 年;Schulte, 2015 年)。氧气和容量限制热耐受性假说 (OCLTT) 提出,整个生物体的热性能受到生物体的代谢需求和供氧能力的限制,当极端温度下的氧气需求超过生物体获取和获取氧气的能力时,就会设定生物体的热极限。循环氧气 (Pörtner, 2001 ; Pörtner, 2002 年)。OCLTT 假设在不同的分类群中得到了不同的支持(Ern 等人, 2016 年;Gangloff 和 Telemeco, 2018 年;Pörtner 和 Knust, 2007 年;Verberk 等人, 2016 年),并引发了关于其生态相关性和普遍性的激烈争论(Clark 等人)等人, 2013 年;Jutfelt 等人, 2018 年;Pörtner 等人, 2018 年)。
最近,开发了热限制分层机制 (HMTL) 假说,以协调支持和驳斥等温动物 OCLTT 的观察结果(框 1;Gangloff 和 Telemeco, 2018 年))。与 OCLTT 类似,HMTL 假设提出,整个有机体的性能是由合适温度下的有氧能力驱动的。然而,HMTL 明确认识到,临界热限制可以通过多种机制设定,包括缺乏有氧能力和亚细胞成分(如蛋白质或膜)的失效。无论是首先达到有氧能力限制还是亚细胞功能限制,都会在近端设置热限制,并且这种层次结构可以随着生活史阶段和环境背景而发生可预测的变化。除了从机械上解释许多等温动物的 TPC 特征的不对称形状外,HMTL 还对 TPC 形状如何受到氧气环境和容量的影响做出了具体预测(方框 1;有关详细信息,请参阅 Gangloff 和 Telemeco, 2018)。原则上,HMTL 预测,当生物体获取或利用氧气的能力受到限制时,即使在热极限,例如临界热最大值 (CT MAX),不受影响。高海拔侵入、屏气、剧烈活动、怀孕、疾病和个体发育阶段是有氧能力降低可能对等温动物的 TPC 形状产生有意义影响的常见情况。因此,HMTL 框架可以提供一个重要的工具来预测有机体对新的或变化的环境的反应。这种关于极端温度在生命史阶段或氧气容量或可用性不同的环境中的亚致死和致死影响的定量预测在全球气候紧急情况所呈现的生态环境中尤其具有先见之明,包括在海水变暖或上坡迁移的背景下(Ern 等人, 2016 年;Jacobsen, 2020 年;Rodgers 等人, 2021 年;Storz, 2021 年)。
框 1.热限制的分级机制
热限制假设的分层机制 (HMTL; Gangloff & Telemeco, 2018 ) 结合了氧气和容量限制热容限假设 (OCLTT; Pörtner, 2001 , 2002 ; Pörtner et al., 2017 )、边际稳定性假设 ( Hochachka & Somero, 2002 ) 和热性能曲线范式 (TPC; Huey & Stevenson, 1979) 机械地描述温度和氧气可用性如何相互作用以影响等温动物的整体性能。图 B1 提供了 HMTL 假设的示意图。与 OCLTT 一样,HMTL 提出整个机体的表现与有氧范围、静息代谢需求和最大代谢能力之间的差异成正比。随着温度升高到临界热最小值 (CT MIN ) 以上,当最大代谢率 (MMR) 等于生物体的最大有氧能力时(图 B1 顶部面板中的水平虚线),有氧范围会在达到稳定之前增加。随着温度升高到性能的最佳热性能(T OPT),MMR 保持不变,但由于热对代谢动力学的影响,静息代谢率 (RMR) 继续增加,从而导致有氧范围减小。这种有氧运动表现的缓慢下降将持续到 RMR 等于 MMR 并且有氧范围等于零(图 B1)。我们将此点称为“有氧临界温度”(有氧 T CRIT)。相比之下,我们将亚细胞成分严重丧失功能的温度称为 “亚细胞 T CRIT ”'。虽然亚细胞过程的破坏可能(并且可能会)导致有氧能力失败,但该过程不同于由有氧能力直接决定的有机体性能限制,如需氧 T CRIT所定义。HMTL 建议 CT MAX由较低的 T CRIT驱动(因此 HMTL 中的“分层机制”)。在 HMTL 下,降低有氧 T CRIT的有氧能力的任何变化都可能导致驱动 CT MAX的亚细胞机制转变为有氧机制。这甚至适用于通常受亚细胞 T CRIT限制的物种,如图 B1c 中的“有氧能力极低”条件所示。HMTL 的主要概念进步是它明确承认氧气限制或边际稳定性可能是高温下性能损失的基础。此外,这种机制可以塑性和可预测地过渡。
当有氧或亚细胞 T CRIT接近设定高温极限时, HMTL 对 TPC 的形状做出了多项新颖且可测试的预测,以及 TPC 形状将如何响应氧气环境或有氧能力的变化而变化。当有氧 T CRIT低于 CT MAX时,HMTL 预测 TPC 将是对称的(图 B1c)。这种对称性是由于需氧范围随着温度高于 T OPT而减小,其速率与需氧范围随着温度低于 T OPT增加的速率相同。然而,当亚细胞 T CRIT是 CT MAX的基础时, HMTL 预测 TPC 将偏斜,这是大多数等温线的典型情况(图 B1a,b)。此外,HMTL 预测这种偏差将随着需氧 T CRIT和亚细胞 T CRIT之间的距离而增加。这种不对称性是由于亚细胞 T CRIT在低于由于需求和供应能力不匹配而导致主要有氧限制的温度下导致快速性能损失的结果。HMTL 提供了一个框架,该框架可用于对临界热极限以及 TPC 形状如何受外温类群和生态环境中的氧气环境影响进行机械预测。

使用 TPC 作为预测工具的另一个挑战是选择相关性状作为整个有机体性能或适应度的代理。TPC 可能因性状而异(Clark 等人, 2013 年;Kellermann 等人, 2019 年;Stevenson 等人, 1985 年),并对温度与氧气等因素的相互作用做出不同的反应。因此,如果要将性能测量用作适应度代理,则必须仔细考虑测量它的特征和上下文。运动表现,特别是蜥蜴的冲刺速度,是一种容易且广泛测量的特征,与多种生态环境相关,包括捕食者逃跑、觅食和同种动物之间的冲突(Gilbert & Miles, 2017 ; Miles, 2004)。然而,当使用这个度量作为适应度代理时,有一些重要的考虑因素(Irschick, 2003)。例如,蜥蜴的短距离冲刺速度几乎完全是无氧的(Bennett & Licht, 1972;Gleeson, 1991)。结果,蜥蜴只能在几秒钟到几分钟内保持冲刺表现,然后它们必须休息并使用有氧呼吸来恢复体内平衡(Gleeson, 1982;Gleeson & Hancock, 2002)。任何应用厌氧 TPC 来预测有机体对温度长期变化(例如气候变化的结果)的反应,都隐含地假设为消化、生长和繁殖等大多数功能提供动力的有氧性能能力至少同样高作为跨温度的厌氧性能。如果这个假设没有得到满足,那么像短跑速度这样的厌氧性状将不能很好地代表整个生物体的表现,而应该首选好氧性状。最近的工作强调有氧范围的生态相关性,作为整体有机体性能的综合衡量标准。有氧范围是静息代谢需求和最大代谢能力之间的差异,它代表动物为有氧过程提供动力的能力(Gangloff & Telemeco, 2018; Pörtner 等人, 2017 年;舒尔特, 2015;赛贝尔和德意志, 2020 年)。用于有氧范围的 TPC 有助于将个体动物的实验室测量结果与更广泛的生物地理模式和预测联系起来(Bozinovic & Pörtner, 2015 年;Clark 等人, 2013 年;Pörtner, 2021 年)。虽然冲刺速度和有氧范围都表现出温度依赖性,但它们的燃料供给方式不同,因此它们对氧气环境变化的反应方式可能不同。因为短跑速度几乎完全由无氧呼吸推动(Bennett & Licht, 1972),预计不会受到氧气供应量急剧减少的限制,而最大有氧能力和有氧范围将受到限制(Storz, 2021 年)。
通过这项研究,我们通过在上坡迁徙的普通壁蜥Podarcis Muralis的背景下测量单个 TPC 的冲刺速度和有氧范围来测试和支持对 HMTL 假设的预测。这种分布广泛的物种在历史上仅限于海拔高达 2,000 米 (ASL),但近年来已观察到在海拔较高的栖息地定居(通常观察到高达 2,300 米的 ASL;Pottier, 2020 , F. Aubret, pers. obs .)。移植实验表明,成年P.壁画蜥蜴在高于其当前范围限制的海拔高度受到氧气限制,有氧范围和性能降低(Bodensteiner、Gangloff 等人, 2021 年;Gangloff 等人, 2019 年))。我们假设冲刺速度和有氧范围的 TPC 在低海拔测量时将表现出大致相似的模式,但有氧范围的 TPC 在高海拔地区会受到缺氧的影响,而冲刺速度将不受影响。具体来说,正如 HMTL 假设所预测的(Gangloff 和 Telemeco, 2018),我们预计最大有氧范围和有氧范围的最佳温度都会在高海拔处降低。这些预测的出现是因为氧气可用性降低导致有氧性能的 TPC 变得更加对称和扁平,最大性能大大降低,峰值性能的最佳温度降低(框 1,图 B1b,图 1)。基于我们的经验观察和 HMTL,我们随后开发了一个数学框架,用于描述和预测温度和环境氧气可用性对有机体性能的双重影响,即温度-氧气性能表面 (TOPS)。最后,我们利用这种方法来预测P.muralis殖民高海拔栖息地的潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号