当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
1,2,3-Triazole and Its Analogues: New Surrogates for Diazo Compounds
Chemical Reviews ( IF 62.1 ) Pub Date : 2022-07-19 , DOI: 10.1021/acs.chemrev.1c00991 Monalisa Akter 1 , Kavuri Rupa 1 , Pazhamalai Anbarasan 1
Chemical Reviews ( IF 62.1 ) Pub Date : 2022-07-19 , DOI: 10.1021/acs.chemrev.1c00991 Monalisa Akter 1 , Kavuri Rupa 1 , Pazhamalai Anbarasan 1
Affiliation
|
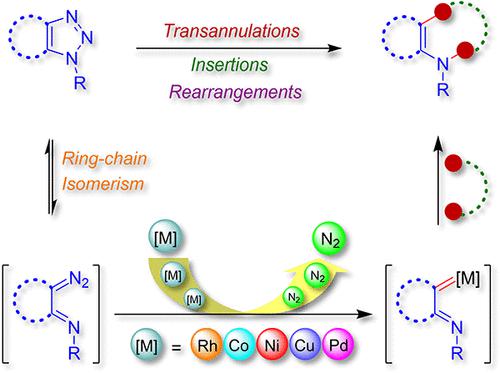
Readily accessible and shelf-stable 1,2,3-triazole and its analogues such as pyridotriazole, triazoloindole, benzotriazole, and thiadiazole exist in equilibrium with their ring-opened isomers, viz., diazo compounds. These ring-opened isomers could be trapped by various metal catalysts (e.g., Rh, Pd, Cu, Co, Ag, etc.) to generate the corresponding metal carbenoids with extrusion of nitrogen. As a consequence, these unique N-heterocycles facilitate access to a realm of N-containing complex structural motifs of biological importance through denitrogenative transformations such as transannulations, insertions, ylide formation, and rearrangements by trapping of the metal carbenoids with a diverse range of coupling partners (e.g., alkenes, alkynes, nitriles, carbo/heterocycles, X–H/C–X bonds, etc.). Hence, suitably substituted triazole derivatives have emerged as efficient surrogates of diazo compounds for the generation of reactive metal carbenoids during the past decades. In this comprehensive review, we aim to discuss in detail the remarkable advancement in their synthesis and synthetic applications.
中文翻译:

1,2,3-三唑及其类似物:重氮化合物的新替代物
容易获得和货架稳定的 1,2,3-三唑及其类似物,如吡啶三唑、三唑并吲哚、苯并三唑和噻二唑与其开环异构体平衡存在,即。, 重氮化合物。这些开环异构体可以被各种金属催化剂(例如,Rh、Pd、Cu、Co、Ag 等)捕获,并在氮的挤压下生成相应的金属类卡宾。因此,这些独特的 N-杂环有助于通过脱氮转化(例如环环化、插入、叶立德形成和重排)进入具有生物学重要性的含 N 复杂结构基序领域,并通过捕获具有多种偶联的金属类卡宾伙伴(例如烯烃、炔烃、腈、碳/杂环、X-H/C-X 键等)。因此,在过去的几十年中,适当取代的三唑衍生物已成为重氮化合物的有效替代物,用于生成反应性金属卡宾。在这次全面审查中,
更新日期:2022-07-19
中文翻译:

1,2,3-三唑及其类似物:重氮化合物的新替代物
容易获得和货架稳定的 1,2,3-三唑及其类似物,如吡啶三唑、三唑并吲哚、苯并三唑和噻二唑与其开环异构体平衡存在,即。, 重氮化合物。这些开环异构体可以被各种金属催化剂(例如,Rh、Pd、Cu、Co、Ag 等)捕获,并在氮的挤压下生成相应的金属类卡宾。因此,这些独特的 N-杂环有助于通过脱氮转化(例如环环化、插入、叶立德形成和重排)进入具有生物学重要性的含 N 复杂结构基序领域,并通过捕获具有多种偶联的金属类卡宾伙伴(例如烯烃、炔烃、腈、碳/杂环、X-H/C-X 键等)。因此,在过去的几十年中,适当取代的三唑衍生物已成为重氮化合物的有效替代物,用于生成反应性金属卡宾。在这次全面审查中,


























 京公网安备 11010802027423号
京公网安备 11010802027423号