Synthetic Metals ( IF 4.4 ) Pub Date : 2022-07-18 , DOI: 10.1016/j.synthmet.2022.117139 Efe Baturhan Orman , Zuhal Yazar , Mehmet Pişkin , Zafer Odabaş , Ali Rıza Özkaya
|
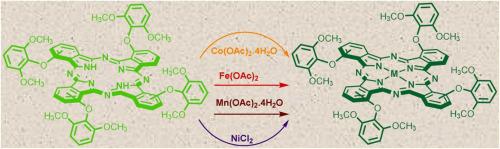
Non-peripherally tetra-2,6-dimethoxyphenoxy substituted Co(II) 3, Fe(II) 4, Mn(III) 5, and Ni(II) 6 phthalocyanines were prepared by refluxing the n-pentanol solution of their metal-free analogue H2Pc 2, (obtained by using 3-(2,6-dimethoxyphenoxy)phthalonitrile), metal salts (Co(CH3COO)2.4 H2O, Fe(CH3COO)2, Mn(CH3COO)2.4 H2O or NiCl2) and 1,8-diazabicyclo[5.4.0]undec-7-ene(DBU) as the catalyst under N2 atmosphere for 2 h. The molecular structure of metallophthalocyanines 3–6 were explained by common methods which are elemental analysis, MALDI-TOF mass, FTIR, and UV-Vis spectroscopies. The complexes are well dissolved in various solvents (dichloromethane, tetrahydrofuran, dimethylsulfoxide, dimethylformamide, and toluene. Electrochemical redox, electrocatalytic oxygen reducing and electrocolorimetric properties of the phthalocyanine complexes were also measured by voltammetric and in situ UV-Vis spectroelectrochemical techniques. The phthalocyanine complexes displayed highly reversible sequential one-electron redox processes occurring at metal and/or phthalocyanine ring. The association of these processes with net colour changes pointed out their functionality as electrochromic material. Furthermore, the phthalocyanine complexes 3-5 and especially Fe(II)Pc 4, showed striking electrocatalytic oxygen reducing activity, owing to the metal centres with redox activity, increasing their interplay with the O2 molecule.
中文翻译:

新型 2,6-二甲氧基苯氧基 α 取代的酞菁金属配合物:电化学、原位光谱电化学和氧电催化
非外围四-2,6-二甲氧基苯氧基取代的Co(II) 3、Fe(II) 4、Mn(III) 5和Ni(II) 6酞菁通过回流它们的无金属的正戊醇溶液制备类似物H 2 Pc 2,(通过使用3-(2,6-二甲氧基苯氧基)邻苯二甲腈获得),金属盐(Co(CH 3 COO)2 .4 H 2 O,Fe(CH 3 COO)2,Mn(CH 3 ) COO) 2 .4 H 2 O 或 NiCl 2 ) 和 1,8-二氮杂双环[5.4.0]undec-7-ene(DBU) 作为 N 2下的催化剂大气 2 小时。金属酞菁3-6的分子结构通过元素分析、MALDI-TOF 质谱、FTIR 和 UV- Vis光谱等常用方法进行了解释。配合物很好地溶解在各种溶剂(二氯甲烷、四氢呋喃、二甲亚砜、二甲基甲酰胺和甲苯)中。还通过伏安法和原位UV- Vis测量了酞菁配合物的电化学氧化还原、电催化氧还原和电比色性质光谱电化学技术。酞菁配合物表现出高度可逆的顺序单电子氧化还原过程,发生在金属和/或酞菁环上。这些过程与净颜色变化的关联表明它们作为电致变色材料的功能。此外,酞菁配合物3-5尤其是 Fe(II)Pc 4显示出惊人的电催化氧还原活性,这是由于金属中心具有氧化还原活性,增加了它们与 O 2分子的相互作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号