Chest ( IF 9.6 ) Pub Date : 2022-07-19 , DOI: 10.1016/j.chest.2022.07.007 Wei-Jie Guan 1 , Jin-Fu Xu 2 , Hong Luo 3 , Xing-Xiang Xu 4 , Yuan-Lin Song 5 , Wan-Li Ma 6 , Zong-An Liang 7 , Xue-Dong Liu 8 , Guo-Jun Zhang 9 , Xiao-Ju Zhang 10 , Rong-Kai Li 11 , Shu-Yang Zhu 12 , Yi-Jie Zhang 13 , Xing-Jun Cai 14 , Li-Ping Wei 15 , Dong-Bo Tian 16 , Hui Zhao 17 , Ping-Yan Chen 18 , Jie-Ming Qu 19 , Nan-Shan Zhong 20 ,
|
Background
Few large-scale studies have demonstrated the efficacy of tobramycin nebulization in bronchiectasis. We evaluated the efficacy and safety of nebulized tobramycin inhalation solution (TIS) in adults with bronchiectasis with Pseudomonas aeruginosa infection.
Research Question
Can TIS effectively reduce sputum P aeruginosa density and improve the bronchiectasis-specific quality of life in patients with bronchiectasis with P aeruginosa infection?
Study Design and Methods
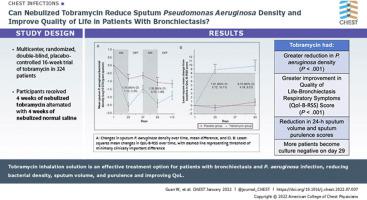
This was a phase 3, 16-week, multicenter, randomized, double-blind, placebo-controlled trial. Eligible adults with bronchiectasis were recruited from October 2018 to July 2021. On the basis of usual care, patients nebulized TIS (300 mg/5 mL twice daily) or normal saline (5 mL twice daily) via vibrating-mesh nebulizer. Treatment consisted of two cycles, each consisting of 28 days on-treatment and 28 days off-treatment. The coprimary end points included changes from baseline in P aeruginosa density and Quality-of-Life Bronchiectasis Respiratory Symptoms score on day 29.
Results
The modified intention-to-treat population consisted of 167 patients in the tobramycin group and 172 patients in the placebo group. Compared with placebo, TIS resulted in a significantly greater reduction in P aeruginosa density (adjusted mean difference, 1.74 log10 colony-forming units/g; 95% CI, 1.12-2.35; P < .001) and greater improvement in Quality-of-Life Bronchiectasis Respiratory Symptoms score (adjusted mean difference, 7.91; 95% CI, 5.72-10.11; P < .001) on day 29. Similar findings were observed on day 85. TIS resulted in a significant reduction in 24-h sputum volume and sputum purulence score on days 29, 57, and 85. More patients became culture negative for P aeruginosa in the tobramycin group than in the placebo group on day 29 (29.3% vs 10.6%). The incidence of adverse events and serious adverse events were comparable between the two groups.
Interpretation
TIS is an effective treatment option and has an acceptable safety profile in patients with bronchiectasis with P aeruginosa infection.
Trial Registration
ClinicalTrials.gov; No. NCT03715322; URL: www.clinicaltrials.gov
中文翻译:

妥布霉素吸入溶液治疗铜绿假单胞菌感染支气管扩张症成人的双盲随机安慰剂对照 3 期试验
背景
很少有大规模研究证明妥布霉素雾化治疗支气管扩张的疗效。我们评估了雾化妥布霉素吸入溶液 (TIS) 在成人支气管扩张伴铜绿假单胞菌感染中的疗效和安全性。
研究问题
TIS能否有效降低痰液铜绿假单胞菌浓度,改善支气管扩张伴铜绿假单胞菌感染患者的支气管扩张特异性生活质量?
研究设计和方法
这是一项为期 16 周的 3 期多中心、随机、双盲、安慰剂对照试验。从2018年10月至2021年7月招募了符合条件的患有支气管扩张症的成人。在常规护理的基础上,患者通过振网雾化器雾化TIS(300 mg / 5 mL,每天两次)或生理盐水(5 mL,每天两次)。治疗包括两个周期,每个周期包括 28 天治疗和 28 天停止治疗。共同主要终点包括第 29 天铜绿假单胞菌密度和生活质量支气管扩张呼吸道症状评分相对于基线的变化。
结果
改良意向治疗人群包括妥布霉素组 167 名患者和安慰剂组 172 名患者。与安慰剂相比,TIS 导致铜绿假单胞菌密度显着降低(调整后的平均差,1.74 log 10菌落形成单位/g;95% CI,1.12-2.35;P < .001),并且质量改善更大- 第 29 天的生命支气管扩张呼吸系统症状评分(调整后的平均差异,7.91;95% CI,5.72-10.11;P < .001)。在第 85 天观察到类似的结果。TIS 导致 24 小时痰量显着减少和第 29、57 和 85 天的痰液化脓评分。更多患者的铜绿假单胞菌培养呈阴性在第 29 天,妥布霉素组比安慰剂组高(29.3% 对 10.6%)。两组不良事件和严重不良事件的发生率具有可比性。
解释
TIS 是一种有效的治疗选择,在铜绿假单胞菌感染的支气管扩张患者中具有可接受的安全性。
试用注册
临床试验.gov;编号 NCT03715322;网址:www.clinicaltrials.gov


























 京公网安备 11010802027423号
京公网安备 11010802027423号