American Journal of Kidney Diseases ( IF 13.2 ) Pub Date : 2022-07-14 , DOI: 10.1053/j.ajkd.2022.05.012 Mini Michael 1 , Jaap W Groothoff 2 , Hadas Shasha-Lavsky 3 , John C Lieske 4 , Yaacov Frishberg 5 , Eva Simkova 6 , Anne-Laure Sellier-Leclerc 7 , Arnaud Devresse 8 , Fitsum Guebre-Egziabher 9 , Sevcan A Bakkaloglu 10 , Chebl Mourani 11 , Rola Saqan 12 , Richard Singer 13 , Richard Willey 14 , Bahru Habtemariam 14 , John M Gansner 14 , Ishir Bhan 14 , Tracy McGregor 14 , Daniella Magen 15
|
Rationale & Objective
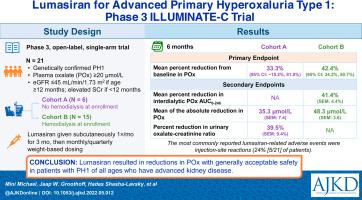
Lumasiran reduces urinary and plasma oxalate (POx) in patients with primary hyperoxaluria type 1 (PH1) and relatively preserved kidney function. ILLUMINATE-C evaluates the efficacy, safety, pharmacokinetics, and pharmacodynamics of lumasiran in patients with PH1 and advanced kidney disease.
Study Design
Phase 3, open-label, single-arm trial.
Setting & Participants
Multinational study; enrolled patients with PH1 of all ages, estimated glomerular filtration rate ≤45 mL/min/1.73 m2 (if age ≥12 months) or increased serum creatinine level (if age <12 months), and POx ≥20 μmol/L at screening, including patients with or without systemic oxalosis.
Intervention
Lumasiran administered subcutaneously; 3 monthly doses followed by monthly or quarterly weight-based dosing.
Outcome
Primary end point: percent change in POx from baseline to month 6 (cohort A; not receiving hemodialysis at enrollment) and percent change in predialysis POx from baseline to month 6 (cohort B; receiving hemodialysis at enrollment). Pharmacodynamic secondary end points: percent change in POx area under the curve between dialysis sessions (cohort B only); absolute change in POx; percent and absolute change in spot urinary oxalate-creatinine ratio; and 24-hour urinary oxalate adjusted for body surface area.
Results
All patients (N = 21; 43% female; 76% White) completed the 6-month primary analysis period. Median age at consent was 8 (range, 0-59) years. For the primary end point, least-squares mean reductions in POx were 33.3% (95% CI, −15.2% to 81.8%) in cohort A (n = 6) and 42.4% (95% CI, 34.2%-50.7%) in cohort B (n = 15). Improvements were also observed in all pharmacodynamic secondary end points. Most adverse events were mild or moderate. No patient discontinued treatment or withdrew from the study. The most commonly reported lumasiran-related adverse events were injection-site reactions, all of which were mild and transient.
Limitations
Single-arm study without placebo control.
Conclusions
Lumasiran resulted in substantial reductions in POx with acceptable safety in patients with PH1 who have advanced kidney disease, supporting its efficacy and safety in this patient population.
Funding
Alnylam Pharmaceuticals.
Trial Registration
Registered at ClinicalTrials.gov with study number NCT04152200 and at EudraCT with study number 2019-001346-17.
Plain-Language Summary
Primary hyperoxaluria type 1 (PH1) is a rare genetic disease characterized by excessive hepatic oxalate production that frequently causes kidney failure. Lumasiran is an RNA interference therapeutic that is administered subcutaneously for the treatment of PH1. Lumasiran has been shown to reduce oxalate levels in the urine and plasma of patients with PH1 who have relatively preserved kidney function. In the ILLUMINATE-C study, the efficacy and safety of lumasiran were evaluated in patients with PH1 and advanced kidney disease, including a cohort of patients undergoing hemodialysis. During the 6-month primary analysis period, lumasiran resulted in substantial reductions in plasma oxalate with acceptable safety in patients with PH1 complicated by advanced kidney disease.
中文翻译:

用于晚期原发性高草酸尿症 1 型的 Lumasiran:3 期 ILLUMINATE-C 试验
理由和目标
Lumasiran 可降低原发性高草酸尿症 1 型 (PH1) 患者的尿液和血浆草酸盐 (POx) 且肾功能相对保留。ILLUMINATE-C 评估了 lumasiran 在 PH1 和晚期肾病患者中的疗效、安全性、药代动力学和药效学。
学习规划
第 3 阶段,开放标签,单臂试验。
设置和参与者
跨国学习;入组所有年龄段的 PH1 患者,估计肾小球滤过率≤45 mL/min/1.73 m 2(如果年龄≥12 个月)或血清肌酐水平升高(如果年龄<12 个月),筛选时 POx ≥20 μmol/L ,包括有或没有全身性草酸中毒的患者。
干涉
Lumasiran 皮下给药;3 个月一次的剂量,然后是每月或每季度基于体重的剂量。
结果
主要终点:POx 从基线到第 6 个月的百分比变化(队列 A;入组时未接受血液透析)和透析前 POx 从基线到第 6 个月的百分比变化(队列 B;入组时接受血液透析)。药效学次要终点:透析期间曲线下 POx 面积的百分比变化(仅队列 B);POx 的绝对变化;现场尿草酸肌酐比值的百分比和绝对变化;以及根据体表面积调整的 24 小时尿草酸盐。
结果
所有患者(N = 21;43% 为女性;76% 为白人)完成了 6 个月的主要分析期。同意的中位年龄为 8(范围,0-59)岁。对于主要终点,队列 A(n = 6)中 POx 的最小二乘平均减少率为 33.3%(95% CI,-15.2% 至 81.8%)和 42.4%(95% CI,34.2%-50.7%)在队列 B 中(n = 15)。在所有药效学次要终点中也观察到改善。大多数不良事件是轻度或中度的。没有患者停止治疗或退出研究。最常报告的与 lumasiran 相关的不良事件是注射部位反应,所有这些都是轻微和短暂的。
限制
没有安慰剂对照的单臂研究。
结论
Lumasiran 导致患有晚期肾病的 PH1 患者的 POx 显着减少且安全性可接受,支持其在该患者群体中的有效性和安全性。
资金
Alnylam 制药公司。
试用注册
在 ClinicalTrials.gov 注册,研究编号为 NCT04152200,在 EudraCT 注册,研究编号为 2019-001346-17。
通俗易懂的摘要
原发性高草酸尿症 1 型 (PH1) 是一种罕见的遗传病,其特征是肝脏产生过多的草酸盐,经常导致肾衰竭。Lumasiran 是一种 RNA 干扰疗法,皮下注射用于治疗 PH1。Lumasiran 已被证明可以降低肾功能相对保留的 PH1 患者尿液和血浆中的草酸盐水平。在 ILLUMINATE-C 研究中,lumasiran 的疗效和安全性在 PH1 和晚期肾病患者中进行了评估,包括一组接受血液透析的患者。在 6 个月的主要分析期间,lumasiran 导致 PH1 并发晚期肾病患者的血浆草酸盐显着减少,安全性可接受。


























 京公网安备 11010802027423号
京公网安备 11010802027423号