Journal of Advanced Research ( IF 10.7 ) Pub Date : 2022-07-14 , DOI: 10.1016/j.jare.2022.07.002 Mingge Ding 1 , Rui Shi 2 , Feng Fu 3 , Man Li 4 , Dema De 5 , Yanyan Du 4 , Zongfang Li 6
|
Introduction
The anti-cancer medication doxorubicin (Dox) is largely restricted in clinical usage due to its significant cardiotoxicity. The only medication approved by the FDA for Dox-induced cardiotoxicity is dexrazoxane, while it may reduce the sensitivity of cancer cells to chemotherapy and is restricted for use. There is an urgent need for the development of safe and effective medicines to alleviate Dox-induced cardiotoxicity.
Objectives
The objective of this study was to determine whether Paeonol (Pae) has the ability to protect against Dox-induced cardiotoxicity and if so, what are the underlying mechanisms involved.
Methods
Sprague-Dawley rats and primary cardiomyocytes were used to create Dox-induced cardiotoxicity models. Pae's effects on myocardial damage, mitochondrial function, mitochondrial dynamics and signaling pathways were studied using a range of experimental methods.
Results
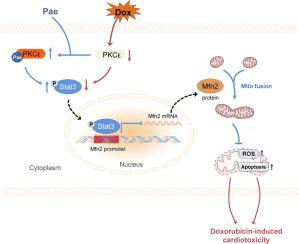
Pae enhanced Mfn2-mediated mitochondrial fusion, restored mitochondrial function and cardiac performance both in vivo and in vitro under the Dox conditions. The protective properties of Pae were blunted when Mfn2 was knocked down or knocked out in Dox-induced cardiomyocytes and hearts respectively. Mechanistically, Pae promoted Mfn2-mediated mitochondria fusion by activating the transcription factor Stat3, which bound to the Mfn2 promoter in a direct manner and up-regulated its transcriptional expression. Furthermore, molecular docking, surface plasmon resonance and co-immunoprecipitation studies showed that Pae’s direct target was PKCε, which interacted with Stat3 and enabled its phosphorylation and activation. Pae-induced Stat3 phosphorylation and Mfn2-mediated mitochondrial fusion were inhibited when PKCε was knocked down. Furthermore, Pae did not interfere with Dox's antitumor efficacy in several tumor cells.
Conclusion
Pae protects the heart against Dox-induced damage by stimulating mitochondrial fusion via the PKCε-Stat3-Mfn2 pathway, indicating that Pae might be a promising therapeutic therapy for Dox-induced cardiotoxicity while maintaining Dox's anticancer activity.
中文翻译:

丹皮酚通过激活 PKCε-Stat3 通路促进 Mfn2 介导的线粒体融合来预防阿霉素诱导的心脏毒性
介绍
由于其显着的心脏毒性,抗癌药物多柔比星 (Dox) 在临床上的使用受到很大限制。FDA 批准用于治疗 Dox 引起的心脏毒性的唯一药物是右雷佐生,但它可能会降低癌细胞对化疗的敏感性,因此限制使用。迫切需要开发安全有效的药物来减轻 Dox 引起的心脏毒性。
目标
本研究的目的是确定丹皮酚 (Pae) 是否有能力防止 Dox 引起的心脏毒性,如果有,涉及的潜在机制是什么。
方法
Sprague-Dawley 大鼠和原代心肌细胞用于创建 Dox 诱导的心脏毒性模型。使用一系列实验方法研究了 Pae 对心肌损伤、线粒体功能、线粒体动力学和信号通路的影响。
结果
Pae 增强了 Mfn2 介导的线粒体融合,在体内和体外恢复了线粒体功能和心脏性能在 Dox 条件下。当 Mfn2 在 Dox 诱导的心肌细胞和心脏中分别被敲低或敲除时,Pae 的保护特性减弱。从机制上讲,Pae 通过激活转录因子 Stat3 促进 Mfn2 介导的线粒体融合,Stat3 以直接方式与 Mfn2 启动子结合并上调其转录表达。此外,分子对接、表面等离子体共振和共免疫沉淀研究表明,Pae 的直接靶标是 PKCε,它与 Stat3 相互作用并使其磷酸化和激活。当 PKCε 被敲低时,Pae 诱导的 Stat3 磷酸化和 Mfn2 介导的线粒体融合被抑制。此外,Pae 不会干扰 Dox 在几种肿瘤细胞中的抗肿瘤功效。
结论
Pae 通过 PKCε-Stat3-Mfn2 通路刺激线粒体融合来保护心脏免受 Dox 诱导的损伤,表明 Pae 可能是一种有希望的治疗 Dox 诱导的心脏毒性的疗法,同时保持 Dox 的抗癌活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号