Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Bacterial chemotaxis in static gradients quantified in a biopolymer membrane-integrated microfluidic platform
Lab on a Chip ( IF 6.1 ) Pub Date : 2022-07-11 , DOI: 10.1039/d2lc00481j Piao Hu 1 , Khanh L Ly 2 , Le P H Pham 1 , Alex E Pottash 3 , Kathleen Sheridan 2 , Hsuan-Chen Wu 4 , Chen-Yu Tsao 4 , David Quan 4 , William E Bentley 3, 4 , Gary W Rubloff 5 , Herman O Sintim 6 , Xiaolong Luo 1
Lab on a Chip ( IF 6.1 ) Pub Date : 2022-07-11 , DOI: 10.1039/d2lc00481j Piao Hu 1 , Khanh L Ly 2 , Le P H Pham 1 , Alex E Pottash 3 , Kathleen Sheridan 2 , Hsuan-Chen Wu 4 , Chen-Yu Tsao 4 , David Quan 4 , William E Bentley 3, 4 , Gary W Rubloff 5 , Herman O Sintim 6 , Xiaolong Luo 1
Affiliation
|
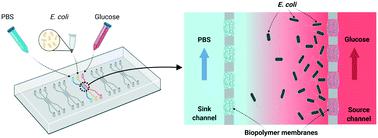
Chemotaxis is a fundamental bacterial response mechanism to changes in chemical gradients of specific molecules known as chemoattractant or chemorepellent. The advancement of biological platforms for bacterial chemotaxis research is of significant interest for a wide range of biological and environmental studies. Many microfluidic devices have been developed for its study, but challenges still remain that can obscure analysis. For example, cell migration can be compromised by flow-induced shear stress, and bacterial motility can be impaired by nonspecific cell adhesion to microchannels. Also, devices can be complicated, expensive, and hard to assemble. We address these issues with a three-channel microfluidic platform integrated with natural biopolymer membranes that are assembled in situ. This provides several unique attributes. First, a static, steady and robust chemoattractant gradient was generated and maintained. Second, because the assembly incorporates assembly pillars, the assembled membrane arrays connecting nearby pillars can be created longer than the viewing window, enabling a wide 2D area for study. Third, the in situ assembled biopolymer membranes minimize pressure and/or chemiosmotic gradients that could induce flow and obscure chemotaxis study. Finally, nonspecific cell adhesion is avoided by priming the polydimethylsiloxane (PDMS) microchannel surfaces with Pluronic F-127. We demonstrated chemotactic migration of Escherichia coli as well as Pseudomonas aeruginosa under well-controlled easy-to-assemble glucose gradients. We characterized motility using the chemotaxis partition coefficient (CPC) and chemotaxis migration coefficient (CMC) and found our results consistent with other reports. Further, random walk trajectories of individual cells in simple bright field images were conveniently tracked and presented in rose plots. Velocities were calculated, again in agreement with previous literature. We believe the biopolymer membrane-integrated platform represents a facile and convenient system for robust quantitative assessment of cellular motility in response to various chemical cues.
中文翻译:

在生物聚合物膜集成微流体平台中量化静态梯度中的细菌趋化性
趋化性是细菌对特定分子化学梯度变化的基本反应机制,称为化学引诱剂或化学排斥剂。用于细菌趋化性研究的生物平台的进步对于广泛的生物和环境研究具有重要意义。许多微流体设备已为其研究开发,但仍然存在可能掩盖分析的挑战。例如,细胞迁移可能会受到流动诱导的剪切应力的影响,而细菌运动可能会因非特异性细胞粘附到微通道而受损。此外,设备可能很复杂、昂贵且难以组装。我们通过与原位组装的天然生物聚合物膜集成的三通道微流体平台来解决这些问题. 这提供了几个独特的属性。首先,生成并维持静态、稳定和稳健的趋化梯度。其次,由于该组件包含组装柱,连接附近柱的组装膜阵列可以创建得比观察窗更长,从而为研究提供了广阔的二维区域。第三,原位组装的生物聚合物膜最大限度地减少了可能诱导流动和模糊趋化性研究的压力和/或化学渗透梯度。最后,通过使用 Pluronic F-127 启动聚二甲基硅氧烷 (PDMS) 微通道表面,可以避免非特异性细胞粘附。我们展示了大肠杆菌和铜绿假单胞菌的趋化性迁移在控制良好的易于组装的葡萄糖梯度下。我们使用趋化性分配系数 (CPC) 和趋化性迁移系数 (CMC) 对运动进行了表征,发现我们的结果与其他报告一致。此外,简单明场图像中单个细胞的随机游走轨迹被方便地跟踪并呈现在玫瑰图中。计算速度,再次与以前的文献一致。我们相信生物聚合物膜集成平台代表了一种简单方便的系统,可以对响应各种化学信号的细胞运动进行稳健的定量评估。
更新日期:2022-07-11
中文翻译:

在生物聚合物膜集成微流体平台中量化静态梯度中的细菌趋化性
趋化性是细菌对特定分子化学梯度变化的基本反应机制,称为化学引诱剂或化学排斥剂。用于细菌趋化性研究的生物平台的进步对于广泛的生物和环境研究具有重要意义。许多微流体设备已为其研究开发,但仍然存在可能掩盖分析的挑战。例如,细胞迁移可能会受到流动诱导的剪切应力的影响,而细菌运动可能会因非特异性细胞粘附到微通道而受损。此外,设备可能很复杂、昂贵且难以组装。我们通过与原位组装的天然生物聚合物膜集成的三通道微流体平台来解决这些问题. 这提供了几个独特的属性。首先,生成并维持静态、稳定和稳健的趋化梯度。其次,由于该组件包含组装柱,连接附近柱的组装膜阵列可以创建得比观察窗更长,从而为研究提供了广阔的二维区域。第三,原位组装的生物聚合物膜最大限度地减少了可能诱导流动和模糊趋化性研究的压力和/或化学渗透梯度。最后,通过使用 Pluronic F-127 启动聚二甲基硅氧烷 (PDMS) 微通道表面,可以避免非特异性细胞粘附。我们展示了大肠杆菌和铜绿假单胞菌的趋化性迁移在控制良好的易于组装的葡萄糖梯度下。我们使用趋化性分配系数 (CPC) 和趋化性迁移系数 (CMC) 对运动进行了表征,发现我们的结果与其他报告一致。此外,简单明场图像中单个细胞的随机游走轨迹被方便地跟踪并呈现在玫瑰图中。计算速度,再次与以前的文献一致。我们相信生物聚合物膜集成平台代表了一种简单方便的系统,可以对响应各种化学信号的细胞运动进行稳健的定量评估。


























 京公网安备 11010802027423号
京公网安备 11010802027423号