当前位置:
X-MOL 学术
›
Can. J. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Thermodynamic and kinetic study of fluorinated gas hydrates for water purification
The Canadian Journal of Chemical Engineering ( IF 2.1 ) Pub Date : 2022-07-10 , DOI: 10.1002/cjce.24537 Hai Son Truong‐Lam 1, 2 , Changsu Jeon 1 , Ju Dong Lee 1
The Canadian Journal of Chemical Engineering ( IF 2.1 ) Pub Date : 2022-07-10 , DOI: 10.1002/cjce.24537 Hai Son Truong‐Lam 1, 2 , Changsu Jeon 1 , Ju Dong Lee 1
Affiliation
|
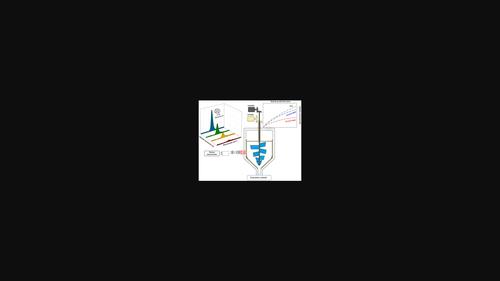
This study investigated and compared the thermodynamic stability, kinetic behaviour, and effectiveness of a water purification process using pentafluoroethane (HFC125a) and 1,1,1,2-tetrafluoroethane (HFC134a) as guest molecules. The hydrate phase equilibria of each fluorinated gas (F-gas) in pure water and NaCl solution were predicted using the Hu-Lee-Sum correlation, which agreed well with the experimental results from our previous studies. Under the same subcooling temperature of 3 K (at 0.3 MPa), the rate of hydrate growth with HFC134a was faster than that of HFC125a in the absence or presence of NaCl. In situ Raman spectroscopy confirmed that the HFC134a and HFC125a molecules occupy only a large cage of structure II hydrate. The Raman shifts of CH and CC bands in all phases (gas, liquid, and hydrate phases) of HFC125a shifted to higher wavelengths than those of HFC134a due to the increase in the number of fluorine atoms. The change in the salinity was studied to evaluate the effectiveness of an F-gas hydrate-based water purification process. In addition, the desalination efficiency of the HFC134a and HFC125a hydrates was compared by separating hydrate crystals from the slurries. The results showed that the desalination efficiency (or total dissolved solids removal efficiency) of HFC134a hydrate was higher than that of HFC125a hydrate. This study proves the importance of the water purification process using hydrates.
中文翻译:

水净化用氟化气体水合物的热力学和动力学研究
本研究调查并比较了使用五氟乙烷 (HFC125a) 和 1,1,1,2-四氟乙烷 (HFC134a) 作为客体分子的水净化过程的热力学稳定性、动力学行为和有效性。使用 Hu-Lee-Sum 相关性预测了纯水和 NaCl 溶液中每种氟化气体 (F-gas) 的水合物相平衡,这与我们之前研究的实验结果非常吻合。在 3 K(0.3 MPa)的相同过冷温度下,在不存在或存在 NaCl 的情况下,HFC134a 的水合物生长速率快于 HFC125a。原位拉曼光谱证实 HFC134a 和 HFC125a 分子仅占据结构 II 水合物的大笼。C H 和 C 的拉曼位移由于氟原子数量的增加,HFC125a 的所有相(气相、液相和水合物相)中的 C 带移动到比 HFC134a 更高的波长。研究了盐度的变化,以评估基于含氟气体水合物的水净化工艺的有效性。此外,通过从浆液中分离水合物晶体,比较了 HFC134a 和 HFC125a 水合物的脱盐效率。结果表明,HFC134a水合物的脱盐效率(或总溶解固体去除效率)高于HFC125a水合物。这项研究证明了使用水合物的水净化过程的重要性。
更新日期:2022-07-10
中文翻译:

水净化用氟化气体水合物的热力学和动力学研究
本研究调查并比较了使用五氟乙烷 (HFC125a) 和 1,1,1,2-四氟乙烷 (HFC134a) 作为客体分子的水净化过程的热力学稳定性、动力学行为和有效性。使用 Hu-Lee-Sum 相关性预测了纯水和 NaCl 溶液中每种氟化气体 (F-gas) 的水合物相平衡,这与我们之前研究的实验结果非常吻合。在 3 K(0.3 MPa)的相同过冷温度下,在不存在或存在 NaCl 的情况下,HFC134a 的水合物生长速率快于 HFC125a。原位拉曼光谱证实 HFC134a 和 HFC125a 分子仅占据结构 II 水合物的大笼。C H 和 C 的拉曼位移由于氟原子数量的增加,HFC125a 的所有相(气相、液相和水合物相)中的 C 带移动到比 HFC134a 更高的波长。研究了盐度的变化,以评估基于含氟气体水合物的水净化工艺的有效性。此外,通过从浆液中分离水合物晶体,比较了 HFC134a 和 HFC125a 水合物的脱盐效率。结果表明,HFC134a水合物的脱盐效率(或总溶解固体去除效率)高于HFC125a水合物。这项研究证明了使用水合物的水净化过程的重要性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号