Journal of Chromatography B ( IF 3 ) Pub Date : 2022-07-05 , DOI: 10.1016/j.jchromb.2022.123363 Amira S Gouda 1 , Hoda M Marzouk 2 , Mamdouh R Rezk 2 , Ahmed M Salem 3 , Mosaad I Morsi 1 , Eman G Nouman 1 , Youmna M Abdallah 1 , Ahmed Y Hassan 1 , Ahmed M Abdel-Megied 4
|
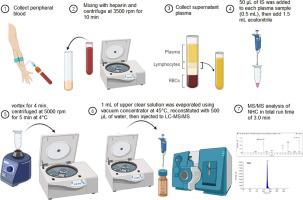
A fully validated, simple, rapid and reproducible liquid chromatography-tandem mass spectrometry method was developed to determine NHC (N-hydroxycytidine), the active metabolite of Molnupiravir (MOL) in human plasma; one of the limited treatment options for SARS-CoV-2 in plasma of healthy volunteers. The internal standard (IS) used was ribavirin. The extraction of analyte and IS from plasma was performed using acetonitrile as a solvent for protein precipitation. Agilent Zorbax Eclipse plus C18, 4.6 × 150 mm, (5 µm) was used for chromatographic separation using a mixture of methanol0.2 % acetic acid (5:95, v/v) as a mobile phase that was pumped at a flow rate of 0.9 mL/min. Detection was performed on a triple quadrupole mass spectrometer operating in multiple reaction monitoring (MRM) employing positive ESI interface using API4500 triple quadrupole tandem mass spectrometer system, with the transitions set at m/z 260.10 → 128.10 and 245.10 → 113.20 for NHC and IS respectively. Method validation was performed in accordance with United States FDA bioanalytical guidance. The concentration range of 20.0–10000.0 ng/mL was used to establish linearity via weighted linear regression approach (1/x2). Moreover, the analyzed pharmacokinetic data from twelve Egyptian healthy volunteers were used to develop a population pharmacokinetic model for NHC. The developed model was used to perform simulations and evaluate the current MOL dosing recommendations through calculating the maximum concentration (Cmax) “the safety metric” and area under the curve (AUC0-12 h) “the efficacy metric” for 1000 virtual subjects. Geometric mean ratios (GMR) with their associated 90% confidence intervals (CI) compared to literature values were computed. Geometric means of simulation-based Cmax and AUC0-12 were 3827 ng/mL (GMR = 1.05; 90% CI = 0.96–1.15) and 9320 ng.h/mL (GMR = 1.04; 90% CI = 0.97–1.11), respectively indicating that current MOL dosage can achieve the therapeutic targets and dose adjustment may not be required for the Egyptian population. The developed model could be used in the future to refine MOL dosage once further therapeutic targets are identified.
中文翻译:

一种经过验证的 LC-MS/MS 方法,用于测定人血浆中的抗病毒前药 molnupiravir 及其在健康埃及志愿者药代动力学模型研究中的应用
开发了一种经过充分验证、简单、快速和可重现的液相色谱-串联质谱法来测定 NHC(N-羟基胞苷),即人血浆中 Molnupiravir (MOL) 的活性代谢物;健康志愿者血浆中 SARS-CoV-2 的有限治疗选择之一。使用的内标 (IS) 是利巴韦林。使用乙腈作为蛋白质沉淀溶剂,从血浆中提取分析物和内标。Agilent Zorbax Eclipse plus C 18, 4.6 × 150 mm, (5 µm) 用于色谱分离,使用 methanol0.2% 乙酸 (5:95, v/v) 的混合物作为流动相,以 0.9 mL/min 的流速泵送. 使用 API4500 三重四极串联质谱仪系统在采用正 ESI 接口的多反应监测 (MRM) 操作的三重四极质谱仪上进行检测,NHC 和 IS 的跃迁分别设置为m / z 260.10 → 128.10 和 245.10 → 113.20 . 方法验证是根据美国 FDA 生物分析指南进行的。20.0–10000.0 ng/mL 的浓度范围用于通过加权线性回归方法 (1/x 2). 此外,来自 12 名埃及健康志愿者的分析药代动力学数据被用于开发 NHC 的群体药代动力学模型。开发的模型用于通过计算最大浓度(C max)“安全指标”和曲线下面积(AUC 0-12 h) 1000 个虚拟对象的“功效指标”。计算了与文献值相比的几何平均比率 (GMR) 及其相关的 90% 置信区间 (CI)。基于模拟的 Cmax 和 AUC0-12 的几何平均值为 3827 ng/mL(GMR = 1.05;90% CI = 0.96–1.15)和 9320 ng.h/mL(GMR = 1.04;90% CI = 0.97–1.11),分别表明目前的 MOL 剂量可以达到治疗目标,埃及人群可能不需要调整剂量。一旦确定了进一步的治疗目标,所开发的模型可以在未来用于改进 MOL 剂量。



























 京公网安备 11010802027423号
京公网安备 11010802027423号