当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of boron-doped carbon nanotubes by thermocatalytic decomposition of ethanol using a floating catalyst chemical vapor deposition method: kinetic study
Reaction Chemistry & Engineering ( IF 3.9 ) Pub Date : 2022-07-04 , DOI: 10.1039/d1re00536g Shrilekha V. Sawant 1 , Kinshuk Dasgupta 2, 3 , Jyeshtharaj B. Joshi 2, 3 , Ashwin W. Patwardhan 1
Reaction Chemistry & Engineering ( IF 3.9 ) Pub Date : 2022-07-04 , DOI: 10.1039/d1re00536g Shrilekha V. Sawant 1 , Kinshuk Dasgupta 2, 3 , Jyeshtharaj B. Joshi 2, 3 , Ashwin W. Patwardhan 1
Affiliation
|
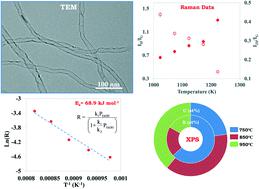
A kinetic study was conducted to define experimental rates of reaction for the production of boron-doped carbon nanotubes (BCNTs) using a floating catalyst chemical vapor deposition technique. Here, boric acid (boron) and ferrocene (catalyst) dissolved in ethanol (carbon) were used as precursors. As a part of the kinetic study, the synthesis temperature, partial pressure of the boron precursor, catalyst precursor, and carbon source were studied in detail. A seven step reaction mechanism and a rate expression were proposed. Using the developed reaction kinetic model, we proposed the irreversible adsorption of ethanol followed by abstraction of hydrogen from the ethyl group adsorbed on iron nanoparticles to be the rate-controlling step. From the Arrhenius plot, in the temperature range of 750–950 °C, the activation energy for the overall reaction was found to be 68.9 kJ mol−1. It was found that an increase in synthesis temperature is responsible for the reduction in boron content (at%) and also defect density. Various spectroscopic and microscopic analyses were performed to evaluate the properties, quality, and purity of BCNTs formed.
中文翻译:

使用浮动催化剂化学气相沉积法热催化分解乙醇合成硼掺杂碳纳米管:动力学研究
进行了一项动力学研究,以确定使用浮动催化剂化学气相沉积技术生产掺硼碳纳米管 (BCNT) 的实验反应速率。这里,溶解在乙醇(碳)中的硼酸(硼)和二茂铁(催化剂)用作前体。作为动力学研究的一部分,详细研究了硼前体、催化剂前体和碳源的合成温度、分压。提出了七步反应机理和速率表达式。使用开发的反应动力学模型,我们提出了乙醇的不可逆吸附,然后从吸附在铁纳米颗粒上的乙基中提取氢作为速率控制步骤。根据 Arrhenius 图,在 750–950 °C 的温度范围内,-1。发现合成温度的升高是硼含量(at%)降低以及缺陷密度降低的原因。进行了各种光谱和显微镜分析以评估形成的 BCNT 的性质、质量和纯度。
更新日期:2022-07-04
中文翻译:

使用浮动催化剂化学气相沉积法热催化分解乙醇合成硼掺杂碳纳米管:动力学研究
进行了一项动力学研究,以确定使用浮动催化剂化学气相沉积技术生产掺硼碳纳米管 (BCNT) 的实验反应速率。这里,溶解在乙醇(碳)中的硼酸(硼)和二茂铁(催化剂)用作前体。作为动力学研究的一部分,详细研究了硼前体、催化剂前体和碳源的合成温度、分压。提出了七步反应机理和速率表达式。使用开发的反应动力学模型,我们提出了乙醇的不可逆吸附,然后从吸附在铁纳米颗粒上的乙基中提取氢作为速率控制步骤。根据 Arrhenius 图,在 750–950 °C 的温度范围内,-1。发现合成温度的升高是硼含量(at%)降低以及缺陷密度降低的原因。进行了各种光谱和显微镜分析以评估形成的 BCNT 的性质、质量和纯度。



























 京公网安备 11010802027423号
京公网安备 11010802027423号