当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalysis and Electron Transfer in De Novo Designed Metalloproteins
Chemical Reviews ( IF 62.1 ) Pub Date : 2022-06-28 , DOI: 10.1021/acs.chemrev.1c01025 Karl J Koebke 1 , Tyler B J Pinter 1 , Winston C Pitts 1 , Vincent L Pecoraro 1
Chemical Reviews ( IF 62.1 ) Pub Date : 2022-06-28 , DOI: 10.1021/acs.chemrev.1c01025 Karl J Koebke 1 , Tyler B J Pinter 1 , Winston C Pitts 1 , Vincent L Pecoraro 1
Affiliation
|
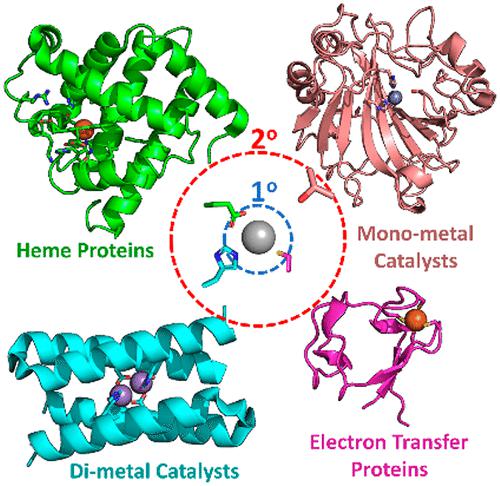
One of the hallmark advances in our understanding of metalloprotein function is showcased in our ability to design new, non-native, catalytically active protein scaffolds. This review highlights progress and milestone achievements in the field of de novo metalloprotein design focused on reports from the past decade with special emphasis on de novo designs couched within common subfields of bioinorganic study: heme binding proteins, monometal- and dimetal-containing catalytic sites, and metal-containing electron transfer sites. Within each subfield, we highlight several of what we have identified as significant and important contributions to either our understanding of that subfield or de novo metalloprotein design as a discipline. These reports are placed in context both historically and scientifically. General suggestions for future directions that we feel will be important to advance our understanding or accelerate discovery are discussed.
中文翻译:

从头设计的金属蛋白中的催化和电子转移
我们对金属蛋白功能理解的标志性进展之一体现在我们设计新的、非天然的、具有催化活性的蛋白质支架的能力上。这篇综述重点介绍了从头金属蛋白设计领域的进展和里程碑式成就,重点关注过去十年的报告,特别强调生物无机研究的常见子领域中的从头设计:血红素结合蛋白、含单金属和二金属的催化位点,和含金属的电子转移位点。在每个子领域中,我们强调了一些我们认为对我们对该子领域的理解或从头理解具有重要意义的贡献金属蛋白设计作为一门学科。这些报告置于历史和科学背景下。讨论了我们认为对于促进我们的理解或加速发现很重要的未来方向的一般建议。
更新日期:2022-06-28
中文翻译:

从头设计的金属蛋白中的催化和电子转移
我们对金属蛋白功能理解的标志性进展之一体现在我们设计新的、非天然的、具有催化活性的蛋白质支架的能力上。这篇综述重点介绍了从头金属蛋白设计领域的进展和里程碑式成就,重点关注过去十年的报告,特别强调生物无机研究的常见子领域中的从头设计:血红素结合蛋白、含单金属和二金属的催化位点,和含金属的电子转移位点。在每个子领域中,我们强调了一些我们认为对我们对该子领域的理解或从头理解具有重要意义的贡献金属蛋白设计作为一门学科。这些报告置于历史和科学背景下。讨论了我们认为对于促进我们的理解或加速发现很重要的未来方向的一般建议。



























 京公网安备 11010802027423号
京公网安备 11010802027423号