Water Research ( IF 12.8 ) Pub Date : 2022-06-26 , DOI: 10.1016/j.watres.2022.118796 Ruiyang Qin 1 , Shuai Chang 1 , Jian Mei 1 , Qianqian Hong 1 , Shijian Yang 1
|
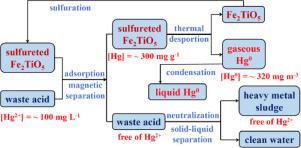
The selective removal of Hg2+ from waste acids containing high concentrations of other metal cations, such as Cu2+, Zn2+, and Cd2+, which are discharged from nonferrous metal smelting industries, is in great demand. Herein, sulfureted Fe2TiO5 was developed as a regenerable magnetic sorbent to recover Hg2+ from waste acids for centralized control. Sulfureted Fe2TiO5 exhibited an excellent ability for Hg2+ removal with the capacity of 292–317 mg g−1 and the rate of 49.5–57.6 mg g−1 h−1 at pH=2–4. Meanwhile, it exhibited an excellent selectivity for Hg2+ removal that not only the coexisting Cu2+, Zn2+, and Cd2+ can scarcely be adsorbed but also Hg2+ adsorption was hardly inhibited. The mechanism and kinetic studies indicated that the Fe2+ in the FeS2 coated on sulfureted Fe2TiO5 was exchanged with Hg2+ adsorbed at a Fe2+ to Hg2+ mole ratio of 1:2. Meanwhile, most of the Hg2+ removed by sulfureted Fe2TiO5 can be thermally desorbed primarily as ultra-high concentrations of gaseous Hg0, which can finally be recovered as liquid Hg0 for centralized control in combination with existing Hg0-recovery devices in smelters. Moreover, the spent sulfureted Fe2TiO5 could be regenerated for duty-cycle operations with re-sulfuration without a remarkable degradation of the Hg2+-removal performance. Therefore, Hg2+ recovery using sulfureted Fe2TiO5 may be a promising, low-cost, and environmentally friendly technology for the centralized control of Hg2+ in waste acids discharged from smelters.
中文翻译:

硫化 Fe2TiO5 选择性去除酸性废水中的 Hg2+:潜在机理及其作为可再生吸附剂从冶炼厂废酸中回收 Hg 的应用
从有色金属冶炼行业排放的含有高浓度其他金属阳离子如Cu 2+、Zn 2+和Cd 2+的废酸中选择性去除Hg 2+的需求很大。本文开发了硫化Fe 2 TiO 5作为可再生磁性吸附剂从废酸中回收Hg 2+以进行集中控制。硫化Fe 2 TiO 5表现出优异的Hg 2+去除能力,容量为292-317 mg g -1,速率为49.5-57.6 mg g -1 h -1在 pH = 2–4 时。同时,它表现出优异的Hg 2+去除选择性,不仅难以吸附共存的Cu 2+、Zn 2+和Cd 2+ ,而且几乎不抑制Hg 2+的吸附。机理和动力学研究表明,硫化Fe 2 TiO 5上包覆的FeS 2中的Fe 2+与吸附的Hg 2+ 交换,Fe 2+与Hg 2+的摩尔比为1:2。同时,硫化Fe 2 TiO 5去除的大部分Hg 2+主要可热解吸为超高浓度的气态Hg 0,最终可回收为液态Hg 0 ,与冶炼厂现有的Hg 0回收装置相结合进行集中控制。此外,用过的硫化Fe 2 TiO 5可以再生用于具有再硫化的工作循环操作,而不会显着降低Hg 2+去除性能。因此,硫化Fe 2 TiO 5回收Hg 2+可能是集中控制冶炼厂排放废酸中Hg 2+的一种有前景的、低成本、环保的技术。



























 京公网安备 11010802027423号
京公网安备 11010802027423号