当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Explaining Regiodivergent Vinylations with Vinylbenziodoxolones**
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2022-06-24 , DOI: 10.1002/anie.202206347 Ester M Di Tommaso 1 , Per-Ola Norrby 2 , Berit Olofsson 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2022-06-24 , DOI: 10.1002/anie.202206347 Ester M Di Tommaso 1 , Per-Ola Norrby 2 , Berit Olofsson 1
Affiliation
|
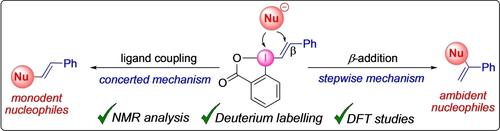
The first mechanistic investigation of vinylbenziodoxolone (VBX) vinylations is reported, using NMR studies, deuterium labelling and computations. Monodent nucleophiles react with VBX through a ligand coupling pathway to give internal alkenes, whereas terminal alkenes form with ambident nucleophiles through a Michael-type addition mechanism. The findings can be used to predict the regioselectivity in VBX vinylations of other nucleophiles.
中文翻译:

用 Vinylbenziodoxolones 解释区域发散乙烯基化**
首次使用 NMR 研究、氘标记和计算对乙烯基苯并氧唑酮 (VBX) 乙烯基化进行了机理研究。单齿亲核试剂通过配体偶联途径与 VBX 反应生成内部烯烃,而末端烯烃通过迈克尔型加成机制与周围的亲核试剂形成。这些发现可用于预测其他亲核试剂在 VBX 乙烯基化中的区域选择性。
更新日期:2022-06-24
中文翻译:

用 Vinylbenziodoxolones 解释区域发散乙烯基化**
首次使用 NMR 研究、氘标记和计算对乙烯基苯并氧唑酮 (VBX) 乙烯基化进行了机理研究。单齿亲核试剂通过配体偶联途径与 VBX 反应生成内部烯烃,而末端烯烃通过迈克尔型加成机制与周围的亲核试剂形成。这些发现可用于预测其他亲核试剂在 VBX 乙烯基化中的区域选择性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号