当前位置:
X-MOL 学术
›
Inorg. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rapid “self-healing” behavior induced by chloride anions to renew the Fe–Ni(oxy)hydroxide surface for long-term alkaline seawater electrolysis
Inorganic Chemistry Frontiers ( IF 7 ) Pub Date : 2022-06-24 , DOI: 10.1039/d2qi01078j Ruo-Yao Fan 1 , Xin-Yu Zhang 1 , Ning Yu 1 , Feng-Ge Wang 1 , Hui-Ying Zhao 1 , Xin Liu 1 , Qian-Xi Lv 1 , Da-Peng Liu 1 , Yong-Ming Chai 1 , Bin Dong 1
Inorganic Chemistry Frontiers ( IF 7 ) Pub Date : 2022-06-24 , DOI: 10.1039/d2qi01078j Ruo-Yao Fan 1 , Xin-Yu Zhang 1 , Ning Yu 1 , Feng-Ge Wang 1 , Hui-Ying Zhao 1 , Xin Liu 1 , Qian-Xi Lv 1 , Da-Peng Liu 1 , Yong-Ming Chai 1 , Bin Dong 1
Affiliation
|
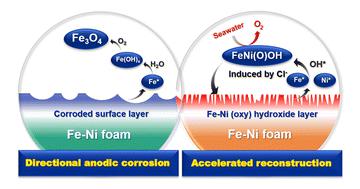
Due to the surface adsorption and interlayer insertion behavior of chloride anions, the Fe–Ni(oxy)hydroxide catalytic surface is easily destroyed, making it difficult to be used for long-term seawater electrolysis. Here, we developed a time- and labor-saving rapid surface reconstruction assisted by a directional anodic corrosion strategy to in situ construct a chrysanthemum shaped active catalytic layer of Fe–Ni(oxy)hydroxide used for seawater electrolysis on the surface of commercial self-supported metal materials. The purpose of the initial artificial anodic corrosion process is to directionally control the rapid corrosion of Fe on the surface of Fe–Ni foam to form a relatively stable Ni-rich structure, which can act as the host of Fe* (Fe* or Ni* represent surface active species of high-valence generated by the oxidation activation) redeposition to slow down the loss of active Fe*. Due to the special structure of the Fe–Ni(oxy)hydroxide catalytic layer tightly loaded on the surface of the Fe–Ni alloy, during the later stability test in natural seawater, the Cl− corrosion can accelerate the surface reconstruction of Fe–Ni(oxy)hydroxide to cause “rapid self-healing”, which is not only conducive to the regular renewal of the catalytic layer, but can prolong the stability of its resistance to seawater electrolysis. Eventually, the sample prepared by anodic corrosion and electrochemical reconstruction (named AC-FeNi(O)OH) only required 252 mV of low overpotential to provide a current density of 100 mA cm−2, and also tolerated over 100 hours in alkaline brine and natural seawater.
中文翻译:

氯离子诱导的快速“自愈”行为更新 Fe-Ni(oxy)hydroxide 表面,用于长期碱性海水电解
由于氯离子的表面吸附和层间插入行为,Fe-Ni(氧)氢氧化物催化表面容易被破坏,难以用于长期的海水电解。在这里,我们开发了一种省时省力的快速表面重建方法,该方法由定向阳极腐蚀策略辅助原位在商用自支撑金属材料表面构建用于海水电解的菊花状 Fe-Ni(oxy)hydroxide 活性催化层。初始人工阳极腐蚀过程的目的是定向控制 Fe 在 Fe-Ni 泡沫表面的快速腐蚀,形成相对稳定的富 Ni 结构,可以作为 Fe*(Fe* 或 Ni * 表示由氧化活化产生的高价表面活性物质)再沉积以减缓活性 Fe* 的损失。由于 Fe-Ni(oxy)hydroxide 催化层的特殊结构紧密负载在 Fe-Ni 合金表面,在后期的天然海水稳定性测试中,Cl -腐蚀可以加速Fe-Ni(oxy)hydroxide的表面重构,引起“快速自愈”,不仅有利于催化层的定期更新,而且可以延长其耐海水电解的稳定性。最终,通过阳极腐蚀和电化学重建制备的样品(命名为 AC-FeNi(O)OH)仅需要 252 mV 的低过电势即可提供 100 mA cm -2的电流密度,并且在碱性盐水中耐受超过 100 小时和天然海水。
更新日期:2022-06-24
中文翻译:

氯离子诱导的快速“自愈”行为更新 Fe-Ni(oxy)hydroxide 表面,用于长期碱性海水电解
由于氯离子的表面吸附和层间插入行为,Fe-Ni(氧)氢氧化物催化表面容易被破坏,难以用于长期的海水电解。在这里,我们开发了一种省时省力的快速表面重建方法,该方法由定向阳极腐蚀策略辅助原位在商用自支撑金属材料表面构建用于海水电解的菊花状 Fe-Ni(oxy)hydroxide 活性催化层。初始人工阳极腐蚀过程的目的是定向控制 Fe 在 Fe-Ni 泡沫表面的快速腐蚀,形成相对稳定的富 Ni 结构,可以作为 Fe*(Fe* 或 Ni * 表示由氧化活化产生的高价表面活性物质)再沉积以减缓活性 Fe* 的损失。由于 Fe-Ni(oxy)hydroxide 催化层的特殊结构紧密负载在 Fe-Ni 合金表面,在后期的天然海水稳定性测试中,Cl -腐蚀可以加速Fe-Ni(oxy)hydroxide的表面重构,引起“快速自愈”,不仅有利于催化层的定期更新,而且可以延长其耐海水电解的稳定性。最终,通过阳极腐蚀和电化学重建制备的样品(命名为 AC-FeNi(O)OH)仅需要 252 mV 的低过电势即可提供 100 mA cm -2的电流密度,并且在碱性盐水中耐受超过 100 小时和天然海水。



























 京公网安备 11010802027423号
京公网安备 11010802027423号