当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Substrate access path-guided engineering of L-threonine aldolase for improving diastereoselectivity
Chemical Communications ( IF 4.9 ) Pub Date : 2022-06-24 , DOI: 10.1039/d2cc02644a Wenlong Zheng 1 , Zhongji Pu 1 , Lanxin Xiao 2 , Gang Xu 2 , Lirong Yang 1, 2 , Haoran Yu 1, 2 , Jianping Wu 1, 2
Chemical Communications ( IF 4.9 ) Pub Date : 2022-06-24 , DOI: 10.1039/d2cc02644a Wenlong Zheng 1 , Zhongji Pu 1 , Lanxin Xiao 2 , Gang Xu 2 , Lirong Yang 1, 2 , Haoran Yu 1, 2 , Jianping Wu 1, 2
Affiliation
|
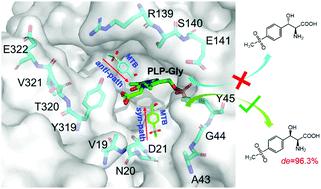
The L-threonine aldolase from Leishmania major was engineered to improve its diastereoselectivity by a CAST/ISM strategy, providing insights into the relationship between the physico-chemical properties of the substrate access path and diastereoselectivity. The steric hindrance, hydrophobic interaction and π–π interaction cooperated to improve the diastereoselectivity of the enzyme, with a diastereomeric excess (de) value reaching 96.3%syn from 26.8%syn.
中文翻译:

用于提高非对映选择性的 L-苏氨酸醛缩酶的底物访问路径引导工程
来自Leishmania major的L-苏氨酸醛缩酶被设计为通过 CAST/ISM 策略提高其非对映选择性,从而深入了解底物访问路径的物理化学性质与非对映选择性之间的关系。空间位阻、疏水相互作用和 π-π 相互作用共同提高了酶的非对映选择性,非对映体过量 (de) 值从 26.8% syn达到 96.3% syn。
更新日期:2022-06-24
中文翻译:

用于提高非对映选择性的 L-苏氨酸醛缩酶的底物访问路径引导工程
来自Leishmania major的L-苏氨酸醛缩酶被设计为通过 CAST/ISM 策略提高其非对映选择性,从而深入了解底物访问路径的物理化学性质与非对映选择性之间的关系。空间位阻、疏水相互作用和 π-π 相互作用共同提高了酶的非对映选择性,非对映体过量 (de) 值从 26.8% syn达到 96.3% syn。


























 京公网安备 11010802027423号
京公网安备 11010802027423号