当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enantioselective Conjugate Addition of Boronic Acids to α,β-Unsaturated 2-Acyl Imidazoles Catalyzed by Chiral Diols
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2022-06-24 , DOI: 10.1021/acs.joc.2c00929 Guo-Li Chai 1 , Ping Zhang 1 , En-Ze Yao 1 , Junbiao Chang 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2022-06-24 , DOI: 10.1021/acs.joc.2c00929 Guo-Li Chai 1 , Ping Zhang 1 , En-Ze Yao 1 , Junbiao Chang 1
Affiliation
|
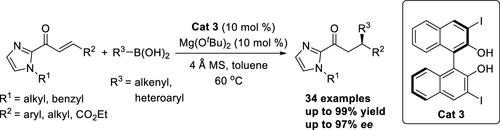
Herein, we report the enantioselective conjugate addition of organic boronic acids to α,β-unsaturated 2-acyl imidazoles using (R)-3,3′-I2-BINOL as the catalyst. The catalytic system shows high efficiency and tolerance to alkenylboronic acids and heteroarylboronic acids. The corresponding Michael addition products were obtained in moderate to excellent yields and with moderate to excellent enantioselectivities (up to 97% ee). A gram-scale reaction was also conducted, and the desired product was obtained in high yield with no erosion in enantioselectivity. Finally, the synthetic utility of the methodology was demonstrated by transforming the 2-acyl imidazole moiety to the corresponding aldehyde, carboxylic acid, ester, and amide derivatives.
中文翻译:

手性二醇催化硼酸与α,β-不饱和2-酰基咪唑的对映选择性共轭加成
在此,我们报道了使用 ( R )-3,3'-I 2 -BINOL 作为催化剂,有机硼酸与 α,β-不饱和 2-酰基咪唑的对映选择性共轭加成。该催化体系显示出高效和对烯基硼酸和杂芳基硼酸的耐受性。相应的迈克尔加成产物以中等至优异的产率和中等至优异的对映选择性(高达 97% ee)获得。还进行了克级反应,并以高收率获得所需产物,而对映选择性没有受到侵蚀。最后,通过将 2-酰基咪唑部分转化为相应的醛、羧酸、酯和酰胺衍生物,证明了该方法的合成效用。
更新日期:2022-06-24
中文翻译:

手性二醇催化硼酸与α,β-不饱和2-酰基咪唑的对映选择性共轭加成
在此,我们报道了使用 ( R )-3,3'-I 2 -BINOL 作为催化剂,有机硼酸与 α,β-不饱和 2-酰基咪唑的对映选择性共轭加成。该催化体系显示出高效和对烯基硼酸和杂芳基硼酸的耐受性。相应的迈克尔加成产物以中等至优异的产率和中等至优异的对映选择性(高达 97% ee)获得。还进行了克级反应,并以高收率获得所需产物,而对映选择性没有受到侵蚀。最后,通过将 2-酰基咪唑部分转化为相应的醛、羧酸、酯和酰胺衍生物,证明了该方法的合成效用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号