当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Li8MnO6: A Novel Cathode Material with Only Anionic Redox
ACS Applied Materials & Interfaces ( IF 9.5 ) Pub Date : 2022-06-23 , DOI: 10.1021/acsami.2c06173 Ningjing Luo 1 , Lianggang Feng 1 , Huimin Yin 1 , Andreas Stein 2 , Shuping Huang 1, 3 , Zhufeng Hou 4 , Donald G Truhlar 2, 5
ACS Applied Materials & Interfaces ( IF 9.5 ) Pub Date : 2022-06-23 , DOI: 10.1021/acsami.2c06173 Ningjing Luo 1 , Lianggang Feng 1 , Huimin Yin 1 , Andreas Stein 2 , Shuping Huang 1, 3 , Zhufeng Hou 4 , Donald G Truhlar 2, 5
Affiliation
|
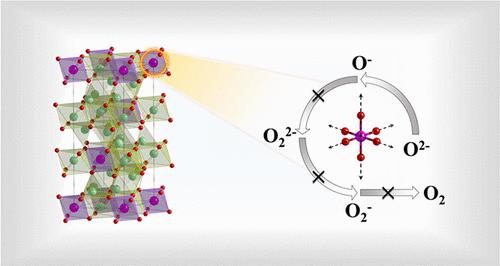
In Li-excess transition metal-oxide cathode materials, anionic oxygen redox can offer high capacity and high voltages, although peroxo and superoxo species may cause oxygen loss, poor cycling performance, and capacity fading. Previous work showed that undesirable formation of peroxide and superoxide bonds was controlled to some extent by Mn substitution, and the present work uses density functional calculations to examine the reasons for this by studying the anionic redox mechanism in Li8MnO6. This material is obtained by substituting Mn for Sn in Li8SnO6 or for Zr in Li8ZrO6, and we also compare this to previous work on those materials. The calculations predict that Li8MnO6 is stable at room temperature (with a band gap of 3.19 eV as calculated by HSE06 and 1.82 eV as calculated with the less reliable PBE+U), and they elucidate the chemical and structural effects involved in the inhibition of oxygen release in this cathode. Throughout the whole delithiation process, only O2– ions are oxidized. The directional Mn–O bonds formed from unfilled 3d orbitals effectively inhibit the formation of O–O bonds, and the layered structure is maintained even after removing 3 Li per Li8MnO6 formula unit. The calculated average voltage for removal of 3 Li is 3.69 V by HSE06, and the corresponding capacity is 389 mAh/g. The high voltage of oxygen anionic redox and the high capacity result in a high energy density of 1436 Wh/kg. The Li-ion diffusion barrier for the dominant interlayer diffusion path along the c axis is 0.57 eV by PBE+U. These results help us to understand the oxygen redox mechanism in a new lithium-rich Li8MnO6 cathode material and contribute to the design of high-energy density lithium-ion battery cathode materials with favorable electrochemical properties based on anionic oxygen redox.
中文翻译:

Li8MnO6:一种只有阴离子氧化还原的新型阴极材料
在锂过量的过渡金属氧化物正极材料中,阴离子氧氧化还原可以提供高容量和高电压,尽管过氧和超氧物质可能会导致氧损失、循环性能差和容量衰减。以前的工作表明,Mn 取代在一定程度上控制了过氧化物和超氧化物键的不良形成,目前的工作通过研究 Li 8 MnO 6中的阴离子氧化还原机制,使用密度泛函计算来检验其原因。这种材料是通过用 Mn 代替 Li 8 SnO 6中的 Sn 或代替 Li 8 ZrO 6中的 Zr 获得的,我们还将其与之前对这些材料的工作进行了比较。计算预测,李8 MnO 6在室温下是稳定的(由 HSE06 计算的带隙为 3.19 eV,由不太可靠的 PBE+U 计算的带隙为 1.82 eV),它们阐明了抑制氧气释放所涉及的化学和结构效应这个阴极。在整个脱锂过程中,只有O 2-离子被氧化。由未填充的 3d 轨道形成的定向 Mn-O 键有效地抑制了 O-O 键的形成,即使在每 Li 8 MnO 6去除 3 Li 后仍保持层状结构公式单位。计算出HSE06去除3 Li的平均电压为3.69 V,对应容量为389 mAh/g。氧阴离子氧化还原的高电压和高容量导致1436 Wh / kg的高能量密度。PBE+U 沿c轴的主要层间扩散路径的锂离子扩散势垒为0.57 eV。这些结果有助于我们了解新型富锂Li 8 MnO 6正极材料中的氧氧化还原机理,并有助于设计基于阴离子氧氧化还原的具有良好电化学性能的高能量密度锂离子电池正极材料。
更新日期:2022-06-23
中文翻译:

Li8MnO6:一种只有阴离子氧化还原的新型阴极材料
在锂过量的过渡金属氧化物正极材料中,阴离子氧氧化还原可以提供高容量和高电压,尽管过氧和超氧物质可能会导致氧损失、循环性能差和容量衰减。以前的工作表明,Mn 取代在一定程度上控制了过氧化物和超氧化物键的不良形成,目前的工作通过研究 Li 8 MnO 6中的阴离子氧化还原机制,使用密度泛函计算来检验其原因。这种材料是通过用 Mn 代替 Li 8 SnO 6中的 Sn 或代替 Li 8 ZrO 6中的 Zr 获得的,我们还将其与之前对这些材料的工作进行了比较。计算预测,李8 MnO 6在室温下是稳定的(由 HSE06 计算的带隙为 3.19 eV,由不太可靠的 PBE+U 计算的带隙为 1.82 eV),它们阐明了抑制氧气释放所涉及的化学和结构效应这个阴极。在整个脱锂过程中,只有O 2-离子被氧化。由未填充的 3d 轨道形成的定向 Mn-O 键有效地抑制了 O-O 键的形成,即使在每 Li 8 MnO 6去除 3 Li 后仍保持层状结构公式单位。计算出HSE06去除3 Li的平均电压为3.69 V,对应容量为389 mAh/g。氧阴离子氧化还原的高电压和高容量导致1436 Wh / kg的高能量密度。PBE+U 沿c轴的主要层间扩散路径的锂离子扩散势垒为0.57 eV。这些结果有助于我们了解新型富锂Li 8 MnO 6正极材料中的氧氧化还原机理,并有助于设计基于阴离子氧氧化还原的具有良好电化学性能的高能量密度锂离子电池正极材料。



























 京公网安备 11010802027423号
京公网安备 11010802027423号