当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Homoallylboration of Aldehydes: Stereoselective Synthesis of Allylic-Substituted Alkenes and E-Alkenes
Organic Letters ( IF 5.2 ) Pub Date : 2022-06-23 , DOI: 10.1021/acs.orglett.2c01789 Daniel Polyak 1 , Bokai Xu 1 , Isaac J Krauss 1
Organic Letters ( IF 5.2 ) Pub Date : 2022-06-23 , DOI: 10.1021/acs.orglett.2c01789 Daniel Polyak 1 , Bokai Xu 1 , Isaac J Krauss 1
Affiliation
|
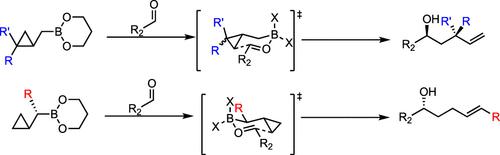
Cyclopropanated allylboration reagents participate in the homoallylation of aliphatic and aromatic aldehydes, generating substituted alkenes that are difficult to produce via other methods. In this study, we explored the scope and reactivity of homoallylation with cyclopropylcarbinylboronates bearing various aliphatic and aromatic α- and γ-substituents. α-Alkyl substituted boronates afforded E-disubstituted alkenyl secondary alcohols in high enantiomeric ratios, while aryl substituents promoted rearrangement. γ-Alkyl substituents all resulted in diastereoselective homoallylation, while aryl substitution changed the outcome to cyclopropylcarbinylation.
中文翻译:

醛的高烯丙基硼化:烯丙基取代烯烃和 E-烯烃的立体选择性合成
环丙烷化烯丙基硼化试剂参与脂肪族和芳香族醛的均烯丙基化,生成难以通过其他方法生产的取代烯烃。在这项研究中,我们探讨了与带有各种脂肪族和芳香族 α- 和 γ- 取代基的环丙基羰基硼酸酯进行均烯丙基化的范围和反应性。α-烷基取代的硼酸酯以高对映体比率提供了 E-二取代的烯基仲醇,而芳基取代基促进了重排。γ-烷基取代基均导致非对映选择性均烯丙基化,而芳基取代将结果改变为环丙基羰基化。
更新日期:2022-06-23
中文翻译:

醛的高烯丙基硼化:烯丙基取代烯烃和 E-烯烃的立体选择性合成
环丙烷化烯丙基硼化试剂参与脂肪族和芳香族醛的均烯丙基化,生成难以通过其他方法生产的取代烯烃。在这项研究中,我们探讨了与带有各种脂肪族和芳香族 α- 和 γ- 取代基的环丙基羰基硼酸酯进行均烯丙基化的范围和反应性。α-烷基取代的硼酸酯以高对映体比率提供了 E-二取代的烯基仲醇,而芳基取代基促进了重排。γ-烷基取代基均导致非对映选择性均烯丙基化,而芳基取代将结果改变为环丙基羰基化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号