当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Mechanistic Switch from Homoallylation to Cyclopropylcarbinylation of Aldehydes
Organic Letters ( IF 5.2 ) Pub Date : 2022-06-23 , DOI: 10.1021/acs.orglett.2c01790 Woojin Lee 1 , Daniel Polyak 2 , Bokai Xu 2 , K N Houk 1 , Isaac J Krauss 2
Organic Letters ( IF 5.2 ) Pub Date : 2022-06-23 , DOI: 10.1021/acs.orglett.2c01790 Woojin Lee 1 , Daniel Polyak 2 , Bokai Xu 2 , K N Houk 1 , Isaac J Krauss 2
Affiliation
|
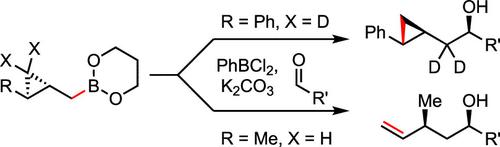
Cyclopropanated allylboration reagents participate in homoallylation reactions of aliphatic and aromatic aldehydes, generating allylic-substituted alkenes that are difficult to produce via other methods. In studying the effect of cyclopropane substituents, we discovered that an aryl substituent completely changes the outcome to cyclopropylcarbinylation, as if the cyclopropylcarbinyl fragment were transferred intact. However, density functional theory computation suggested a novel mechanism involving ring opening and reclosure, which is supported by experimental evidence.
中文翻译:

醛的从同烯丙基化到环丙基羰基化的机制转换
环丙烷化烯丙基硼化试剂参与脂肪族和芳香族醛的均烯丙基化反应,生成难以通过其他方法生产的烯丙基取代烯烃。在研究环丙烷取代基的影响时,我们发现芳基取代基完全改变了环丙基羰基化的结果,就好像环丙基羰基片段被完整转移一样。然而,密度泛函理论计算提出了一种涉及开环和重合的新机制,并得到了实验证据的支持。
更新日期:2022-06-23
中文翻译:

醛的从同烯丙基化到环丙基羰基化的机制转换
环丙烷化烯丙基硼化试剂参与脂肪族和芳香族醛的均烯丙基化反应,生成难以通过其他方法生产的烯丙基取代烯烃。在研究环丙烷取代基的影响时,我们发现芳基取代基完全改变了环丙基羰基化的结果,就好像环丙基羰基片段被完整转移一样。然而,密度泛函理论计算提出了一种涉及开环和重合的新机制,并得到了实验证据的支持。


























 京公网安备 11010802027423号
京公网安备 11010802027423号