当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Study on the Reactive Extraction Process for Heptaldehyde–Undecane–Sodium Bisulfite Aqueous Solution System
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2022-06-22 , DOI: 10.1021/acs.iecr.2c00875 Shenfeng Yuan 1 , Ying Zhang 1 , Zhouna Wan 1 , Hong Yin 1 , Zhirong Chen 1
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2022-06-22 , DOI: 10.1021/acs.iecr.2c00875 Shenfeng Yuan 1 , Ying Zhang 1 , Zhouna Wan 1 , Hong Yin 1 , Zhirong Chen 1
Affiliation
|
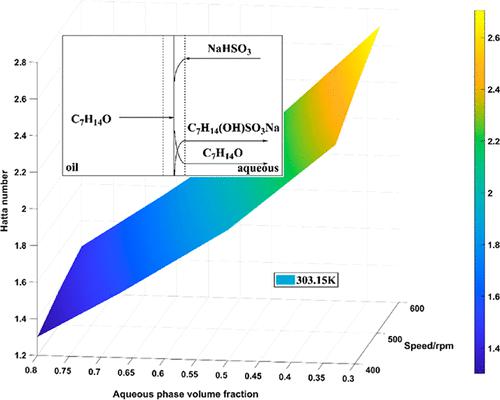
Using sodium bisulfite aqueous solution as extractant, the reactive extraction experiments for a heptaldehyde–undecane mixture were carried out. The results show that when the molar ratio between sodium bisulfite and heptaldehyde, the stirring speed, the volume fraction of aqueous phase or the temperature increase, the reactive extraction rate increases. In contrast, larger molar ratio between sodium bisulfite and heptaldehyde, smaller volume fraction of aqueous phase or lower temperature leads to higher equilibrium conversion of heptaldehyde. According to the mass transfer coefficients of the reactive extraction system and the rate constants obtained from the study of intrinsic kinetics, the Ha numbers were estimated. The results show that under the conditions in this paper, 0.3 < Ha < 3, the reactive extraction process was mainly a mixing control process. Since heptaldehyde is soluble in water while sodium bisulfite is not soluble in undecane, the reaction occurs within the film and the main body of the aqueous phase. For the reactive extraction of 1.0 wt % heptaldehyde in undecane using aqueous sodium bisulfite as the extractant, three methods of performing three-stage reactive extraction were investigated. The results showed that equilibrium extraction is highly necessary to ensure the extraction efficiency. Cross-current equilibrium extraction is suitable for situations where high product purity is required; for situations where the separation requirements are not too high, counter-current equilibrium extraction should be preferred.
中文翻译:

庚醛-十一烷-亚硫酸氢钠水溶液体系的反应萃取工艺研究
使用亚硫酸氢钠水溶液作为萃取剂,进行了庚醛-十一烷混合物的反应萃取实验。结果表明,当亚硫酸氢钠与庚醛的摩尔比、搅拌速度、水相体积分数或温度升高时,反应萃取率增加。相反,较大的亚硫酸氢钠和庚醛摩尔比、较小的水相体积分数或较低的温度导致较高的庚醛平衡转化率。根据反应萃取系统的传质系数和本征动力学研究得到的速率常数,估计了Ha数。结果表明,在本文条件下,0.3 < Ha< 3,反应萃取过程主要是一个混合控制过程。由于庚醛可溶于水,而亚硫酸氢钠不溶于十一烷,因此反应发生在薄膜和水相主体内。对于使用亚硫酸氢钠水溶液作为萃取剂在十一烷中反应萃取 1.0 wt% 庚醛,研究了三种进行三阶段反应萃取的方法。结果表明,平衡萃取对于保证萃取效率是非常必要的。错流平衡萃取适用于产品纯度要求高的场合;对于分离要求不太高的情况,应优先选择逆流平衡萃取。
更新日期:2022-06-22
中文翻译:

庚醛-十一烷-亚硫酸氢钠水溶液体系的反应萃取工艺研究
使用亚硫酸氢钠水溶液作为萃取剂,进行了庚醛-十一烷混合物的反应萃取实验。结果表明,当亚硫酸氢钠与庚醛的摩尔比、搅拌速度、水相体积分数或温度升高时,反应萃取率增加。相反,较大的亚硫酸氢钠和庚醛摩尔比、较小的水相体积分数或较低的温度导致较高的庚醛平衡转化率。根据反应萃取系统的传质系数和本征动力学研究得到的速率常数,估计了Ha数。结果表明,在本文条件下,0.3 < Ha< 3,反应萃取过程主要是一个混合控制过程。由于庚醛可溶于水,而亚硫酸氢钠不溶于十一烷,因此反应发生在薄膜和水相主体内。对于使用亚硫酸氢钠水溶液作为萃取剂在十一烷中反应萃取 1.0 wt% 庚醛,研究了三种进行三阶段反应萃取的方法。结果表明,平衡萃取对于保证萃取效率是非常必要的。错流平衡萃取适用于产品纯度要求高的场合;对于分离要求不太高的情况,应优先选择逆流平衡萃取。


























 京公网安备 11010802027423号
京公网安备 11010802027423号