当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Bromine-radical-induced Csp2–H difluoroalkylation of quinoxalinones and hydrazones through visible-light-promoted Csp3–Br bond homolysis
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-06-23 , DOI: 10.1039/d2qo00710j Chuan-Hua Qu 1 , Run Huang 1 , Yuan Liu 1 , Tong Liu 1 , Gui-Ting Song 1
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-06-23 , DOI: 10.1039/d2qo00710j Chuan-Hua Qu 1 , Run Huang 1 , Yuan Liu 1 , Tong Liu 1 , Gui-Ting Song 1
Affiliation
|
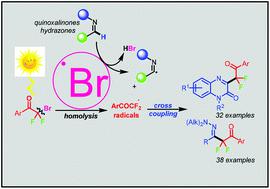
Disclosed herein is a photosensitizer-free strategy for the direct C–H difluoroalkylation of quinoxalinones and hydrazones with bromodifluoroacylarenes via a visible-light-promoted Csp3–Br bond homolytic pathway. This new reaction provides access to difluoroalkylated quinoxaline and hydrazone derivatives with excellent substrate generality under simple, mild, green, and metal-free conditions. This process exploits the fascinating photochemical activity of bromodifluoroacylarenes in the homolysis of Csp3–Br bonds to form difluoroalkyl radicals and bromine radical species. The generated bromine radicals can mediate H abstraction/imine radical formation directly from quinoxalinones and hydrazones, which in turn quench the in situ-generated difluoroalkyl radicals to furnish the products. It is of note is that this is the first example of the application of bromine radicals in organic transformation via Csp3–Br bond homolysis induced by visible light. Moreover, the minimal structural requirements for alkyl bromide-mediated photochemical Csp3–Br bond homolysis were investigated.
中文翻译:

通过可见光促进的 Csp3-Br 键均裂,溴自由基诱导喹喔啉酮和腙的 Csp2-H 二氟烷基化
本文公开了一种不含光敏剂的策略,用于通过可见光促进的 C sp 3 -Br 键均裂途径与溴二氟芳烃直接 C-H 二氟烷基化喹喔啉酮和腙。这种新反应提供了在简单、温和、绿色和无金属条件下获得具有优异底物通用性的二氟烷基化喹喔啉和腙衍生物的途径。该过程利用溴二氟芳烃在 C sp 3 -Br 键均裂形成二氟烷基自由基和溴自由基物种中的迷人光化学活性。产生的溴自由基可以直接从喹喔啉酮和腙中介导 H 提取/亚胺自由基的形成,进而淬灭原位产生的二氟烷基自由基以提供产品。值得注意的是,这是第一个通过可见光诱导的 C sp 3 -Br 键均裂将溴自由基应用于有机转化的例子。此外,研究了烷基溴介导的光化学 C sp 3 -Br 键均裂的最低结构要求。
更新日期:2022-06-23
中文翻译:

通过可见光促进的 Csp3-Br 键均裂,溴自由基诱导喹喔啉酮和腙的 Csp2-H 二氟烷基化
本文公开了一种不含光敏剂的策略,用于通过可见光促进的 C sp 3 -Br 键均裂途径与溴二氟芳烃直接 C-H 二氟烷基化喹喔啉酮和腙。这种新反应提供了在简单、温和、绿色和无金属条件下获得具有优异底物通用性的二氟烷基化喹喔啉和腙衍生物的途径。该过程利用溴二氟芳烃在 C sp 3 -Br 键均裂形成二氟烷基自由基和溴自由基物种中的迷人光化学活性。产生的溴自由基可以直接从喹喔啉酮和腙中介导 H 提取/亚胺自由基的形成,进而淬灭原位产生的二氟烷基自由基以提供产品。值得注意的是,这是第一个通过可见光诱导的 C sp 3 -Br 键均裂将溴自由基应用于有机转化的例子。此外,研究了烷基溴介导的光化学 C sp 3 -Br 键均裂的最低结构要求。


























 京公网安备 11010802027423号
京公网安备 11010802027423号