当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Uncovering the Nature of Band Gap Engineering of Adsorption Energy by Elucidating an Adsorbate Bonding Mechanism on Two-Dimensional TiO2(110)
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2022-06-22 , DOI: 10.1021/acs.jpcc.2c01384 Yameng Liu 1 , Junwei Xu 1 , De-en Jiang 2 , Xiuzhong Fang 1 , Xiang Wang 1 , Xianglan Xu 1
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2022-06-22 , DOI: 10.1021/acs.jpcc.2c01384 Yameng Liu 1 , Junwei Xu 1 , De-en Jiang 2 , Xiuzhong Fang 1 , Xiang Wang 1 , Xianglan Xu 1
Affiliation
|
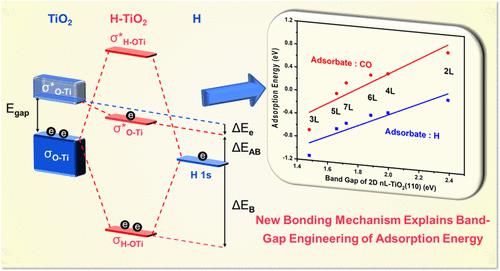
To unravel the nature of band gap engineering of adsorption energy, we investigated the adsorption of H and CO on two-dimensional two-layer (2L)- and three-layer (3L)-TiO2(110) semiconductors by density functional theory calculations. Molecular orbital theory was used to develop a new H–O bonding mechanism. We propose that the H 1s orbital combines linearly with the occupied σO–Ti bonding orbital to form σH–OTi bonding and σ*H–OTi antibonding orbitals. Two of three electrons (one from H and two from σO–Ti) fill the σH–OTi bonding orbital, and the other electron fills the pristine lowest unoccupied σ*O–Ti orbital rather than σ*H–OTi. Consequently, the H adsorption energy EH-ads is decided by the stabilization energies ΔEAB (occupation of σH–OTi) and ΔEe (lowered energy due to electron filling of σ*O–Ti) and the destabilization energy ΔEAB (occupation of σ*O–Ti). The stabilization energies for H adsorption on the 2L and 3L are similar; therefore, the EH-ads difference is predominantly determined by the corresponding ΔEAB, which is dominated by the 2L and 3L band gaps. The C–O bonding mechanism is similar. This mechanism for surface chemical bonding shows the critical role of the band gap in determining the adsorption energy and provides a theoretical basis for band gap engineering in heterogeneous catalysis.
中文翻译:

通过阐明二维 TiO2(110) 上的吸附质键合机制揭示吸附能带隙工程的本质
为了揭示吸附能带隙工程的性质,我们通过密度泛函理论计算研究了 H 和 CO 在二维两层 (2L) 和三层 (3L)-TiO 2 (110) 半导体上的吸附. 分子轨道理论被用来开发一种新的 H-O 键合机制。我们提出 H 1s 轨道与占据的 σ O-Ti键合轨道线性组合,形成 σ H-OTi键合和 σ* H-OTi反键合轨道。三个电子中的两个(一个来自 H,两个来自 σ O-Ti)填充 σ H-OTi键合轨道,另一个电子填充原始最低未占据的 σ* O-Ti轨道,而不是 σ* H-OTi. 因此,H 吸附能E H-ads由稳定能 Δ E AB(占据 σ H-OTi)和 Δ E e(由于 σ* O-Ti的电子填充而降低的能量)和失稳能 Δ E AB(占据 σ* O–Ti)。2L和3L吸附H的稳定能相似;因此,E H-ads差异主要由相应的 Δ E AB决定,主要是 2L 和 3L 带隙。C-O键合机制类似。这种表面化学键合机制显示了带隙在决定吸附能中的关键作用,并为多相催化中的带隙工程提供了理论基础。
更新日期:2022-06-22
中文翻译:

通过阐明二维 TiO2(110) 上的吸附质键合机制揭示吸附能带隙工程的本质
为了揭示吸附能带隙工程的性质,我们通过密度泛函理论计算研究了 H 和 CO 在二维两层 (2L) 和三层 (3L)-TiO 2 (110) 半导体上的吸附. 分子轨道理论被用来开发一种新的 H-O 键合机制。我们提出 H 1s 轨道与占据的 σ O-Ti键合轨道线性组合,形成 σ H-OTi键合和 σ* H-OTi反键合轨道。三个电子中的两个(一个来自 H,两个来自 σ O-Ti)填充 σ H-OTi键合轨道,另一个电子填充原始最低未占据的 σ* O-Ti轨道,而不是 σ* H-OTi. 因此,H 吸附能E H-ads由稳定能 Δ E AB(占据 σ H-OTi)和 Δ E e(由于 σ* O-Ti的电子填充而降低的能量)和失稳能 Δ E AB(占据 σ* O–Ti)。2L和3L吸附H的稳定能相似;因此,E H-ads差异主要由相应的 Δ E AB决定,主要是 2L 和 3L 带隙。C-O键合机制类似。这种表面化学键合机制显示了带隙在决定吸附能中的关键作用,并为多相催化中的带隙工程提供了理论基础。


























 京公网安备 11010802027423号
京公网安备 11010802027423号