当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
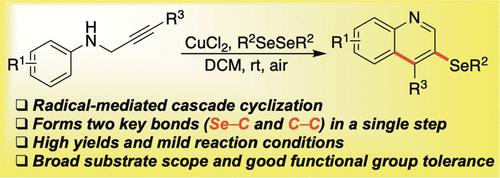
Arylselenyl Radical-Mediated Cyclization of N-(2-Alkynyl)anilines: Access to 3-Selenylquinolines
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2022-06-22 , DOI: 10.1021/acs.joc.2c00282 Changlei Zhu 1 , Max Nurko 1 , Cynthia S Day 1 , John C Lukesh 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2022-06-22 , DOI: 10.1021/acs.joc.2c00282 Changlei Zhu 1 , Max Nurko 1 , Cynthia S Day 1 , John C Lukesh 1
Affiliation
|
An efficient and novel approach to accessing 3-selenylquinolines from diaryl diselenides and acyclic, selenium-free substrates is described. Preliminary mechanistic studies indicate that the combination of CuCl2 and air affords an appropriate environment for producing arylselenyl radicals that initiate the cascade cyclization of N-(2-alkynyl)anilines, forming key Se–C and C–C bonds in a single step. Using this chemistry, a wide variety of 3-selenylquinolines were produced in moderate to excellent yield under mild conditions, highlighting the versatility and usefulness of this new method.
中文翻译:

芳基硒基介导的 N-(2-炔基)苯胺环化:获得 3-硒基喹啉
描述了一种从二芳基二硒化物和无环无硒底物中获取 3-硒基喹啉的有效且新颖的方法。初步机理研究表明,CuCl 2和空气的结合为生成芳基硒基自由基提供了合适的环境,这些自由基引发了N- (2-炔基)苯胺的级联环化,一步形成关键的 Se-C 和 C-C 键。使用这种化学方法,在温和条件下以中等至优异的产率生产了多种 3-硒基喹啉,突出了这种新方法的多功能性和实用性。
更新日期:2022-06-22
中文翻译:

芳基硒基介导的 N-(2-炔基)苯胺环化:获得 3-硒基喹啉
描述了一种从二芳基二硒化物和无环无硒底物中获取 3-硒基喹啉的有效且新颖的方法。初步机理研究表明,CuCl 2和空气的结合为生成芳基硒基自由基提供了合适的环境,这些自由基引发了N- (2-炔基)苯胺的级联环化,一步形成关键的 Se-C 和 C-C 键。使用这种化学方法,在温和条件下以中等至优异的产率生产了多种 3-硒基喹啉,突出了这种新方法的多功能性和实用性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号