当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Stereoselective Access to Iminosugar C,C-Glycosides from 6-Azidoketopyranoses
Organic Letters ( IF 5.2 ) Pub Date : 2022-06-22 , DOI: 10.1021/acs.orglett.2c01560 Zakaria Debbah 1 , Jérôme Marrot 2 , Nicolas Auberger 1 , Jérôme Désiré 1 , Yves Blériot 1
Organic Letters ( IF 5.2 ) Pub Date : 2022-06-22 , DOI: 10.1021/acs.orglett.2c01560 Zakaria Debbah 1 , Jérôme Marrot 2 , Nicolas Auberger 1 , Jérôme Désiré 1 , Yves Blériot 1
Affiliation
|
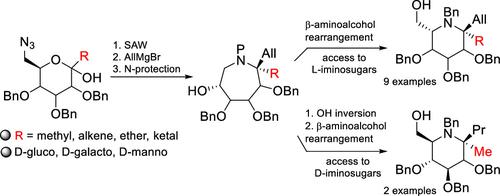
We report the synthesis of iminosugar C,C-glycosides starting from 6-azidoketopyranoses. Their Staudinger-azaWittig-mediated cyclization provided bicyclic N,O-acetals, which were stereoselectively opened with AllMgBr to afford β-hydroxyazepanes with a quaternary carbon α to the nitrogen. Their ring contraction via a β-aminoalcohol rearrangement produced the six-membered l-iminosugars with two functional handles at the pseudoanomeric position. Inversion of the free OH at the azepane level furnished the d-iminosugars.
中文翻译:

从 6-叠氮酮吡喃糖中立体选择性获取亚氨基糖 C,C-糖苷
我们报告了从 6-叠氮酮吡喃糖开始合成亚氨基糖C、C-糖苷。他们的施陶丁格-氮杂维蒂希介导的环化反应提供了双环N , O -缩醛,它们被 AllMgBr 立体选择性地开环,得到了氮上具有季碳 α 的 β-羟基氮杂环己烷。它们通过 β-氨基醇重排的环收缩产生了在假端基位置具有两个功能手柄的六元l-亚氨基糖。氮杂环乙烷水平的游离 OH 的转化提供了d-亚氨基糖。
更新日期:2022-06-22
中文翻译:

从 6-叠氮酮吡喃糖中立体选择性获取亚氨基糖 C,C-糖苷
我们报告了从 6-叠氮酮吡喃糖开始合成亚氨基糖C、C-糖苷。他们的施陶丁格-氮杂维蒂希介导的环化反应提供了双环N , O -缩醛,它们被 AllMgBr 立体选择性地开环,得到了氮上具有季碳 α 的 β-羟基氮杂环己烷。它们通过 β-氨基醇重排的环收缩产生了在假端基位置具有两个功能手柄的六元l-亚氨基糖。氮杂环乙烷水平的游离 OH 的转化提供了d-亚氨基糖。


























 京公网安备 11010802027423号
京公网安备 11010802027423号