当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Trident Molecule with Nanobrush–Nanoparticle–Nanofiber Transition Property Spatially Suppresses Tumor Metastasis
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2022-06-22 , DOI: 10.1021/jacs.2c05743 Ge Gao 1 , Yao-Wen Jiang 2 , Wenjun Zhan 1 , Xiaoyang Liu 1 , Runqun Tang 1 , Xianbao Sun 1 , Yu Deng 1 , Lingling Xu 1 , Gaolin Liang 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2022-06-22 , DOI: 10.1021/jacs.2c05743 Ge Gao 1 , Yao-Wen Jiang 2 , Wenjun Zhan 1 , Xiaoyang Liu 1 , Runqun Tang 1 , Xianbao Sun 1 , Yu Deng 1 , Lingling Xu 1 , Gaolin Liang 1
Affiliation
|
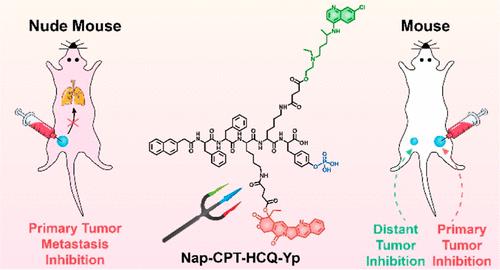
Metastasis-induced high mortality of cancers urgently demands new approaches to simultaneously inhibit primary tumor metastasis and distant tumor growth. Herein, by rational design of a trident molecule Nap–Phe–Phe–Lys(SA-CPT)–Lys(SA-HCQ)–Tyr(H2PO3)–OH (Nap–CPT–HCQ–Yp) with three functional “spears” (i.e., a phosphotyrosine motif for enzymatic self-assembly, camptothecin (CPT) motif for chemotherapy, and hydroxychloroquine (HCQ) motif for autophagy inhibition) and nanobrush–nanoparticle–nanofiber transition property, we propose a novel strategy of intracellular enzymatic nanofiber formation and synergistic autophagy inhibition-enhanced chemotherapy and immunotherapy for spatial suppression of tumor metastasis. Under sequential alkaline phosphatase catalysis and carboxylesterase hydrolysis, Nap–CPT–HCQ–Yp undergoes nanobrush–nanoparticle–nanofiber transition, accompanied by the releases of CPT and HCQ. The formed intracellular nanofibers effectively inhibit the metastasis and invasion behaviors of cancer cells. Meanwhile, the released CPT and HCQ synergistically induce a prominent therapeutic effect through autophagy inhibition-enhanced chemotherapy. Furthermore, chemotherapy of Nap–CPT–HCQ–Yp enhances immunogenic cell death, resulting in the activation of toxic T-cells. Finally, a combination of checkpoint blockade therapy and Nap–CPT–HCQ–Yp-mediated chemotherapy elicits systemic antitumor immunity, thereby achieving efficient inhibitions of primary tumors as well as distant tumors in a breast tumor model. Our work offers a simple and feasible strategy for the design of “smart” multifunctional prodrugs to spatially suppress tumor metastasis.
中文翻译:

具有纳米刷-纳米颗粒-纳米纤维过渡特性的三叉戟分子在空间上抑制肿瘤转移
转移引起的癌症高死亡率迫切需要新的方法来同时抑制原发性肿瘤转移和远处肿瘤的生长。在此,通过合理设计三叉戟分子 Nap-Phe-Phe-Lys(SA-CPT)-Lys(SA-HCQ)-Tyr(H 2 PO 3 )-OH ( Nap - CPT - HCQ - Yp)具有三个功能“矛”(即,用于酶促自组装的磷酸酪氨酸基序、用于化疗的喜树碱(CPT)基序和用于抑制自噬的羟氯喹(HCQ)基序)和纳米刷-纳米颗粒-纳米纤维的转变特性,我们提出了一种新的细胞内酶促纳米纤维形成和协同自噬抑制增强化学疗法和免疫疗法用于空间抑制肿瘤转移的策略。在顺序碱性磷酸酶催化和羧酸酯酶水解下,Nap – CPT – HCQ – Yp经历纳米刷-纳米颗粒-纳米纤维的转变,伴随着CPT和HCQ的释放。形成的细胞内纳米纤维有效抑制癌细胞的转移和侵袭行为。同时,释放的CPT和HCQ通过自噬抑制增强化疗协同诱导显着的治疗效果。此外,Nap – CPT – HCQ – Yp的化学疗法增强了免疫原性细胞死亡,导致毒性 T 细胞活化。最后,检查点封锁疗法和Nap - CPT - HCQ - Yp的组合介导的化学疗法引发全身抗肿瘤免疫,从而在乳腺肿瘤模型中实现对原发性肿瘤和远处肿瘤的有效抑制。我们的工作为设计“智能”多功能前药以空间抑制肿瘤转移提供了一种简单可行的策略。
更新日期:2022-06-22
中文翻译:

具有纳米刷-纳米颗粒-纳米纤维过渡特性的三叉戟分子在空间上抑制肿瘤转移
转移引起的癌症高死亡率迫切需要新的方法来同时抑制原发性肿瘤转移和远处肿瘤的生长。在此,通过合理设计三叉戟分子 Nap-Phe-Phe-Lys(SA-CPT)-Lys(SA-HCQ)-Tyr(H 2 PO 3 )-OH ( Nap - CPT - HCQ - Yp)具有三个功能“矛”(即,用于酶促自组装的磷酸酪氨酸基序、用于化疗的喜树碱(CPT)基序和用于抑制自噬的羟氯喹(HCQ)基序)和纳米刷-纳米颗粒-纳米纤维的转变特性,我们提出了一种新的细胞内酶促纳米纤维形成和协同自噬抑制增强化学疗法和免疫疗法用于空间抑制肿瘤转移的策略。在顺序碱性磷酸酶催化和羧酸酯酶水解下,Nap – CPT – HCQ – Yp经历纳米刷-纳米颗粒-纳米纤维的转变,伴随着CPT和HCQ的释放。形成的细胞内纳米纤维有效抑制癌细胞的转移和侵袭行为。同时,释放的CPT和HCQ通过自噬抑制增强化疗协同诱导显着的治疗效果。此外,Nap – CPT – HCQ – Yp的化学疗法增强了免疫原性细胞死亡,导致毒性 T 细胞活化。最后,检查点封锁疗法和Nap - CPT - HCQ - Yp的组合介导的化学疗法引发全身抗肿瘤免疫,从而在乳腺肿瘤模型中实现对原发性肿瘤和远处肿瘤的有效抑制。我们的工作为设计“智能”多功能前药以空间抑制肿瘤转移提供了一种简单可行的策略。


























 京公网安备 11010802027423号
京公网安备 11010802027423号