Applied Surface Science ( IF 6.7 ) Pub Date : 2022-06-22 , DOI: 10.1016/j.apsusc.2022.154024 Wei Jin , Yingqi Wang , Tong Liu , Changchun Ding , Hua Guo
|
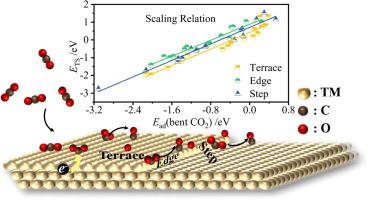
As a promising and practical way to decrease CO2 emissions, the conversion of CO2 to value-added chemicals has received significant recent attention. The activation of CO2 on catalyst surfaces might proceed via a chemisorption state with a bent CO2 configuration, in which substrate electrons are transferred into the antibonding orbital of the CO2 adsorbate. Based on density functional theory calculations, we present an extensive survey of CO2 chemisorption and dissociation on flat and stepped surfaces of several transition metals. The binding energy of the chemisorbed CO2 is closely correlated with the extent of electron transfer from the metal to CO2, as evidenced by a linear relationship found between the CO2 adsorption energy and its Bader charge. Transition state scaling (TSS) correlations between binding energies of transition states and binding energies of either initial or final states are found to exist for the dissociation of the chemisorbed CO2 on flat and stepped surfaces, which can be used to predict the efficacies of the catalysts. Our results show that defect sites at stepped surfaces have a strong influence on CO2 chemical activation and dissociation.
中文翻译:

平面和阶梯过渡金属表面上的 CO2 化学吸附和解离
作为减少CO 2排放的一种有前途且实用的方法,将CO 2 转化为增值化学品最近受到了极大的关注。CO 2在催化剂表面的活化可能通过具有弯曲CO 2构型的化学吸附状态进行,其中底物电子被转移到CO 2吸附物的反键轨道中。基于密度泛函理论计算,我们对几种过渡金属的平面和阶梯表面上的 CO 2化学吸附和解离进行了广泛调查。化学吸附的 CO 2的结合能与电子从金属转移到 CO 的程度密切相关如图2所示,CO 2吸附能与其巴德电荷之间存在线性关系。发现过渡态结合能与初始或最终状态结合能之间存在过渡态标度 (TSS) 相关性,用于化学吸附 CO 2在平坦和阶梯表面上的解离,可用于预测催化剂。我们的结果表明,阶梯表面的缺陷位点对CO 2化学活化和解离有很大的影响。


























 京公网安备 11010802027423号
京公网安备 11010802027423号