当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Role of an Interface for Hydrogen Production Reaction over Size-Controlled Supported Metal Catalysts
ACS Catalysis ( IF 12.9 ) Pub Date : 2022-06-22 , DOI: 10.1021/acscatal.2c02370 Dongjae Shin 1 , Rui Huang 1 , Myeong Gon Jang 1 , Seokhyun Choung 1 , Youngbi Kim 1 , Kiheon Sung 1 , Tae Yong Kim 1 , Jeong Woo Han 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2022-06-22 , DOI: 10.1021/acscatal.2c02370 Dongjae Shin 1 , Rui Huang 1 , Myeong Gon Jang 1 , Seokhyun Choung 1 , Youngbi Kim 1 , Kiheon Sung 1 , Tae Yong Kim 1 , Jeong Woo Han 1
Affiliation
|
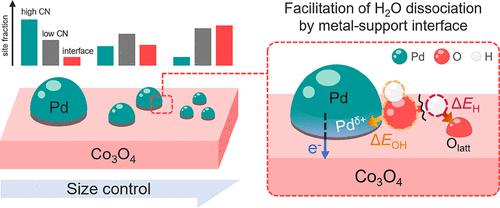
The water–gas shift reaction (WGSR) is important in industries because it can reduce the CO content of syngas to produce purified H2, which can be used as fuel or to make ammonia (NH3). Supported noble metal catalysts have been widely studied for the WGSR because they exhibit high reactivity. However, the role of a metal–support interface in the WGSR has not yet been revealed and remains elusive. Density functional theory (DFT) calculations were performed for a model system of Co3O4-supported Pd (Pd/Co3O4) catalysts. The presence of the interface was found to promote the H2O dissociation step, which is crucial for improving WGSR activity. Thus, the WGSR activity was predicted to be enhanced by an increased number of interfaces, which could be achieved by controlling the size of the supported Pd nanoparticles (NPs). Furthermore, electronic metal–support interactions (MSIs) were found to be a source of the promoted H2O dissociation at the interface. The DFT-predicted promotion of H2O dissociation was further experimentally validated using Pd/Co3O4 catalysts that were size-controlled with calcination temperatures, and the total length of the interface was shown to have a direct correlation with the WGSR rate. Theoretical insights into the role of the interface and the enhancement of WGSR activity due to increased interface sites, which can be achieved by size control, are believed to be useful in the design of efficient supported metal catalysts for the WGSR.
中文翻译:

界面对尺寸控制的负载型金属催化剂的制氢反应的作用
水煤气变换反应 (WGSR) 在工业中很重要,因为它可以降低合成气中的 CO 含量以生产纯化的 H 2,其可用作燃料或制造氨 (NH 3 )。负载型贵金属催化剂已被广泛研究用于 WGSR,因为它们表现出高反应性。然而,金属支撑界面在 WGSR 中的作用尚未揭示并且仍然难以捉摸。对 Co 3 O 4负载的 Pd (Pd/Co 3 O 4 ) 催化剂的模型系统进行了密度泛函理论 (DFT) 计算。发现界面的存在促进了 H 2O 解离步骤,这对于提高 WGSR 活性至关重要。因此,预计 WGSR 活性将通过增加界面数量来增强,这可以通过控制负载的 Pd 纳米颗粒 (NP) 的尺寸来实现。此外,发现电子金属-载体相互作用 (MSI) 是促进 H 2 O 在界面处解离的来源。使用 Pd/Co 3 O 4进一步实验验证了 DFT 预测的 H 2 O 解离促进通过煅烧温度控制尺寸的催化剂,界面的总长度显示与WGSR速率直接相关。对界面作用的理论见解和由于增加的界面位点而提高的 WGSR 活性(可以通过尺寸控制来实现)被认为可用于设计用于 WGSR 的有效负载型金属催化剂。
更新日期:2022-06-22
中文翻译:

界面对尺寸控制的负载型金属催化剂的制氢反应的作用
水煤气变换反应 (WGSR) 在工业中很重要,因为它可以降低合成气中的 CO 含量以生产纯化的 H 2,其可用作燃料或制造氨 (NH 3 )。负载型贵金属催化剂已被广泛研究用于 WGSR,因为它们表现出高反应性。然而,金属支撑界面在 WGSR 中的作用尚未揭示并且仍然难以捉摸。对 Co 3 O 4负载的 Pd (Pd/Co 3 O 4 ) 催化剂的模型系统进行了密度泛函理论 (DFT) 计算。发现界面的存在促进了 H 2O 解离步骤,这对于提高 WGSR 活性至关重要。因此,预计 WGSR 活性将通过增加界面数量来增强,这可以通过控制负载的 Pd 纳米颗粒 (NP) 的尺寸来实现。此外,发现电子金属-载体相互作用 (MSI) 是促进 H 2 O 在界面处解离的来源。使用 Pd/Co 3 O 4进一步实验验证了 DFT 预测的 H 2 O 解离促进通过煅烧温度控制尺寸的催化剂,界面的总长度显示与WGSR速率直接相关。对界面作用的理论见解和由于增加的界面位点而提高的 WGSR 活性(可以通过尺寸控制来实现)被认为可用于设计用于 WGSR 的有效负载型金属催化剂。


























 京公网安备 11010802027423号
京公网安备 11010802027423号