当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The impact of overpotential on the enthalpy of activation and pre-exponential factor of electrochemical redox reactions
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2022-06-22 , DOI: 10.1039/d2cp00404f Anand Kumar Tripathi 1 , Divya Priyadarshani 2 , Miji E Joy 1 , Rajan Maurya 1 , Manoj Neergat 1
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2022-06-22 , DOI: 10.1039/d2cp00404f Anand Kumar Tripathi 1 , Divya Priyadarshani 2 , Miji E Joy 1 , Rajan Maurya 1 , Manoj Neergat 1
Affiliation
|
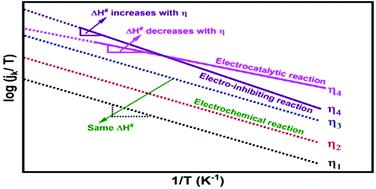
The kinetics of the V5+/V4+ redox reaction is investigated in a three-electrode configuration on a Vulcan XC-72 modified glassy carbon rotating disk electrode at four different temperatures (25 to 40 °C, with 5 °C interval). The values of enthalpy of activation (ΔH#) and pre-exponential factor (Af) estimated using the Eyring equation are in the range of 0.25–0.53 eV (24–51 kJ mol−1) and −1.3 to 5, respectively. The Eyring plots tend to diverge with overpotential, causing an increase in the values of the estimated ΔH# and Af. This is perhaps due to the retarding effect of the precipitates/adsorbates on the electrode surface. The investigation of the kinetics suggests that the V5+/V4+ redox reaction is electrocatalysed through an increase in the entropy of activation (ΔS#).
中文翻译:

过电位对电化学氧化还原反应活化焓和指前因子的影响
V 5+ /V 4+氧化还原反应的动力学在 Vulcan XC-72 改性玻碳旋转圆盘电极上的三电极配置中在四种不同温度(25 至 40 °C,间隔 5 °C)下进行了研究. 使用 Eyring 方程估计的活化焓 (Δ H # ) 和指前因子 ( A f ) 的值分别在 0.25-0.53 eV (24-51 kJ mol -1 ) 和 -1.3 到 5的范围内. 艾林图倾向于随着过电位而发散,导致估计的 Δ H #和A f的值增加. 这可能是由于电极表面上的沉淀物/吸附物的阻滞作用。动力学研究表明,V 5+ /V 4+氧化还原反应是通过增加活化熵(Δ S #)来电催化的。
更新日期:2022-06-22
中文翻译:

过电位对电化学氧化还原反应活化焓和指前因子的影响
V 5+ /V 4+氧化还原反应的动力学在 Vulcan XC-72 改性玻碳旋转圆盘电极上的三电极配置中在四种不同温度(25 至 40 °C,间隔 5 °C)下进行了研究. 使用 Eyring 方程估计的活化焓 (Δ H # ) 和指前因子 ( A f ) 的值分别在 0.25-0.53 eV (24-51 kJ mol -1 ) 和 -1.3 到 5的范围内. 艾林图倾向于随着过电位而发散,导致估计的 Δ H #和A f的值增加. 这可能是由于电极表面上的沉淀物/吸附物的阻滞作用。动力学研究表明,V 5+ /V 4+氧化还原反应是通过增加活化熵(Δ S #)来电催化的。


























 京公网安备 11010802027423号
京公网安备 11010802027423号