当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of Nitrogen-Doped Aza-Helicenes with Chiral Optical Properties
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2022-06-22 , DOI: 10.1021/acs.joc.2c00371 Xue Gong 1 , Chunmei Li 1 , Zhixiong Cai 1 , Xuejuan Wan 1 , Haixia Qian 1 , Guanghui Yang 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2022-06-22 , DOI: 10.1021/acs.joc.2c00371 Xue Gong 1 , Chunmei Li 1 , Zhixiong Cai 1 , Xuejuan Wan 1 , Haixia Qian 1 , Guanghui Yang 1
Affiliation
|
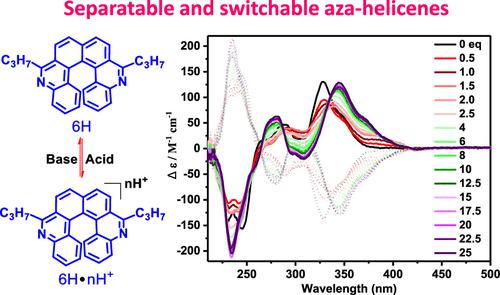
Aza-helicenes are one of the most important series of heterohelicenes; herein, a series of novel aza-helicenes (5H, 6H, 6S, and 8S) were prepared via Bischler–Napieralski cyclization, and the interconversion dynamic process of these aza-helicenes was revealed using density functional theory calculations. The novel nitrogen-doped [6]helicene (6H) possesses a very high interconversion energy barrier of 36.0 kcal/mol. Two enantiomers of 6H were successfully resolved by high-performance liquid chromatography and showed desired chiral optical properties. 6H with chiral optical activity and lone electrons can be a potential candidate for chiral switches, which was demonstrated using the UV and circular dichroism spectra obtained upon titration with an acid and a base.
中文翻译:

具有手性光学性质的氮掺杂氮杂螺旋烯的合成
氮杂-螺旋烯是最重要的杂螺旋烯系列之一;在此,通过Bischler-Napieralski环化制备了一系列新型氮杂-螺旋烯(5H、6H、6S和8S),并利用密度泛函理论计算揭示了这些氮杂-螺旋烯的相互转化动态过程。新型氮掺杂的[6]螺烯( 6H )具有36.0 kcal/mol的非常高的相互转换能垒。6H的两种对映体通过高效液相色谱法成功分离,并显示出所需的手性光学性质。6H具有手性光学活性和孤电子可以成为手性开关的潜在候选者,这通过用酸和碱滴定获得的紫外和圆二色光谱得到证明。
更新日期:2022-06-22
中文翻译:

具有手性光学性质的氮掺杂氮杂螺旋烯的合成
氮杂-螺旋烯是最重要的杂螺旋烯系列之一;在此,通过Bischler-Napieralski环化制备了一系列新型氮杂-螺旋烯(5H、6H、6S和8S),并利用密度泛函理论计算揭示了这些氮杂-螺旋烯的相互转化动态过程。新型氮掺杂的[6]螺烯( 6H )具有36.0 kcal/mol的非常高的相互转换能垒。6H的两种对映体通过高效液相色谱法成功分离,并显示出所需的手性光学性质。6H具有手性光学活性和孤电子可以成为手性开关的潜在候选者,这通过用酸和碱滴定获得的紫外和圆二色光谱得到证明。


























 京公网安备 11010802027423号
京公网安备 11010802027423号