当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
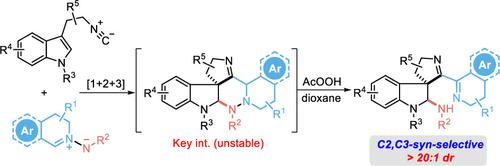
Syn-Stereoselective C3-Spirocyclization and C2-Amination of 3-(2-Isocyanoethyl)indole Using C,N-Cyclic Azomethine Imines
Organic Letters ( IF 5.2 ) Pub Date : 2022-06-22 , DOI: 10.1021/acs.orglett.2c01736 Wen-Bin Cao 1 , Jian-Dong Zhang 1 , Meng-Meng Xu 1 , Hua-Wei Liu 1 , Hai-Yan Li 2 , Xiao-Ping Xu 1, 3 , Shun-Jun Ji 1, 4
Organic Letters ( IF 5.2 ) Pub Date : 2022-06-22 , DOI: 10.1021/acs.orglett.2c01736 Wen-Bin Cao 1 , Jian-Dong Zhang 1 , Meng-Meng Xu 1 , Hua-Wei Liu 1 , Hai-Yan Li 2 , Xiao-Ping Xu 1, 3 , Shun-Jun Ji 1, 4
Affiliation
|
By utilizing an underexplored reaction mode of C,N-cyclic azomethine imines, a catalyst-free [1+2+3] cycloaddition/N–N bond cleavage sequential reaction for accessing spiroindolines with syn-stereoselectivity was developed. On the basis of experimental results and DFT calculations, peroxide and ethereal solvent were identified to trigger the hydrogen abstraction of the unstable [1+2+3] cycloaddition adducts, followed by homolytic cleavage of the N–N bond and hydrogen absorption.
中文翻译:

使用 C,N-环状偶氮甲亚胺对 3-(2-异氰乙基)吲哚进行顺式立体选择性 C3-螺环化和 C2-胺化
通过利用C , N环偶氮甲亚胺的未充分探索的反应模式,开发了一种无催化剂的 [1+2+3] 环加成/N-N 键断裂顺序反应,用于获得具有顺式立体选择性的螺二氢吲哚。根据实验结果和 DFT 计算,确定过氧化物和醚类溶剂会引发不稳定的 [1+2+3] 环加成加合物的夺氢,然后是 N-N 键的均裂和氢吸收。
更新日期:2022-06-22
中文翻译:

使用 C,N-环状偶氮甲亚胺对 3-(2-异氰乙基)吲哚进行顺式立体选择性 C3-螺环化和 C2-胺化
通过利用C , N环偶氮甲亚胺的未充分探索的反应模式,开发了一种无催化剂的 [1+2+3] 环加成/N-N 键断裂顺序反应,用于获得具有顺式立体选择性的螺二氢吲哚。根据实验结果和 DFT 计算,确定过氧化物和醚类溶剂会引发不稳定的 [1+2+3] 环加成加合物的夺氢,然后是 N-N 键的均裂和氢吸收。

























 京公网安备 11010802027423号
京公网安备 11010802027423号