Journal of Hazardous Materials ( IF 13.6 ) Pub Date : 2022-06-20 , DOI: 10.1016/j.jhazmat.2022.129420 Xiaohui Lu 1 , Xiaoqun Zhou 1 , Wei Qiu 1 , Ziyue Wang 1 , Yishi Wang 1 , Haochen Zhang 1 , Jiaxin Yu 1 , Da Wang 2 , Jia Gu 3 , Jun Ma 1
|
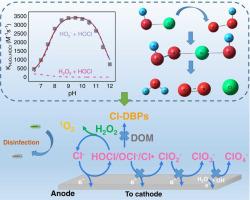
Reduction of HOCl to Cl− by in-situ electrochemical synthesis or ex-situ addition of H2O2 is a feasible method to minimize Cl-DBPs and ClOx− (x = 2, 3, and 4) formation in electrochemical oxidative water treatment systems. This work has investigated the kinetics and mechanism of the reaction between H2O2 and HOCl. The kinetics study showed the species-specific second order rate constants for HOCl with H2O2 (k1), HOCl with HO2− (k2) and OCl− with H2O2 (k3) are 195.5 ± 3.3 M−1s−1, 4.0 × 107 M−1s−1 and 3.5 × 103 M−1s−1, respectively. The density functional theory calculation showed k2 is the most advantageous thermodynamically pathway because it does not need to overcome a high energy barrier. The yields of 1O2 generation from the reaction of H2O2 with HOCl were reinvestigated by using furfuryl alcohol (FFA) as a probe, and an average of 92.3% of 1O2 yields was obtained at pH 7–12. The second order rate constants of the reaction of 1O2 with 13 phenolates were determined by using the H2O2/HOCl system as a quantitative 1O2 production source. To establish a quantitative structure activity relationship, quantum chemical descriptors were more satisfactory than empirical Hammett constants. The potential implications in electrochemical oxidative water treatment were discussed at the end.
中文翻译:

过氧化氢与次氯酸反应的动力学和机理:对电化学水处理的启示
通过原位电化学合成或异位添加 H 2 O 2将 HOCl 还原为 Cl -是减少电化学氧化水中 Cl-DBP 和 ClO x - (x = 2、3 和 4) 形成的可行方法治疗系统。本工作研究了H 2 O 2与HOCl 反应的动力学和机理。动力学研究表明 HOCl 与 H 2 O 2 (k 1 )、HOCl 与 HO 2 - (k 2 ) 和 OCl -与 H 2 O 2 (k )的物种特异性二级速率常数3 )分别为195.5 ± 3.3 M -1 s -1、4.0 × 10 7 M -1 s -1和3.5 × 10 3 M -1 s -1。密度泛函理论计算表明,k 2是最有利的热力学途径,因为它不需要克服高能垒。以糠醇(FFA)为探针,重新研究了H 2 O 2与HOCl反应生成1 O 2的产率,平均1 O 2产率为92.3%。在 pH 7-12 下获得产率。1 O 2与13 种酚盐反应的二级速率常数是通过使用H 2 O 2 /HOCl 体系作为定量1 O 2生产源来确定的。为了建立定量的结构活性关系,量子化学描述符比经验哈米特常数更令人满意。最后讨论了电化学氧化水处理的潜在影响。

























 京公网安备 11010802027423号
京公网安备 11010802027423号