当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
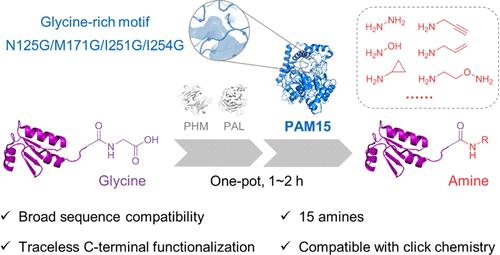
Creating an Unusual Glycine-Rich Motif in a Peptide Amidase Leads to Versatile Protein C-Terminal Traceless Functionalization
ACS Catalysis ( IF 12.9 ) Pub Date : 2022-06-21 , DOI: 10.1021/acscatal.2c01456 Tong Zhu 1, 2 , Yinglu Cui 1 , Wenchao Geng 3 , Guoxia Liu 1 , Huifeng Jiang 3 , Ruifeng Li 1 , Bian Wu 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2022-06-21 , DOI: 10.1021/acscatal.2c01456 Tong Zhu 1, 2 , Yinglu Cui 1 , Wenchao Geng 3 , Guoxia Liu 1 , Huifeng Jiang 3 , Ruifeng Li 1 , Bian Wu 1
Affiliation
|
The rising demand for regioselective protein modifications in chemical biology and pharmaceutical manufacturing has fueled efforts to develop diverse techniques that functionalize native amino acid residues. Although many powerful strategies have provided elegant solutions for functionalizing N-termini and side chains, sequence-unconstrained versatile C-terminal functionalization remains a challenge. Here, we report an engineered peptide amidase (PAM) for C-terminal traceless functionalization in aqueous solution with a broad spectrum of both nucleophiles and protein sequences, excellent yields (up to 98%), and good compatibility with the click reaction. Computational analysis suggested an expanded nucleophile pocket induced by the introduction of an unusual glycine-rich motif, which may enrich the structural diversity in protein design. We anticipate that the successfully engineered PAM holds great potential in the applications of protein chemistry and proteomics, and highlights the employment of serine hydrolases in catalyzing acyl shift reactions that compete with hydrolysis under aqueous conditions.
中文翻译:

在肽酰胺酶中创建不寻常的富含甘氨酸的基序导致多功能蛋白质 C 端无痕功能化
化学生物学和药物制造中对区域选择性蛋白质修饰的需求不断增长,推动了开发多种功能化天然氨基酸残基的技术的努力。尽管许多强大的策略为功能化 N 末端和侧链提供了优雅的解决方案,但序列不受限制的通用 C 末端功能化仍然是一个挑战。在这里,我们报告了一种工程肽酰胺酶 (PAM),用于在水溶液中进行 C 端无痕功能化,具有广谱的亲核试剂和蛋白质序列、优异的产率(高达 98%)以及与点击反应的良好相容性。计算分析表明,通过引入不寻常的富含甘氨酸的基序诱导了一个扩大的亲核试剂袋,这可能丰富了蛋白质设计中的结构多样性。
更新日期:2022-06-21
中文翻译:

在肽酰胺酶中创建不寻常的富含甘氨酸的基序导致多功能蛋白质 C 端无痕功能化
化学生物学和药物制造中对区域选择性蛋白质修饰的需求不断增长,推动了开发多种功能化天然氨基酸残基的技术的努力。尽管许多强大的策略为功能化 N 末端和侧链提供了优雅的解决方案,但序列不受限制的通用 C 末端功能化仍然是一个挑战。在这里,我们报告了一种工程肽酰胺酶 (PAM),用于在水溶液中进行 C 端无痕功能化,具有广谱的亲核试剂和蛋白质序列、优异的产率(高达 98%)以及与点击反应的良好相容性。计算分析表明,通过引入不寻常的富含甘氨酸的基序诱导了一个扩大的亲核试剂袋,这可能丰富了蛋白质设计中的结构多样性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号