Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Affinities and Kinetics Detection of Protein–Small Molecule Interactions with a Monolayer MoS2-Based Optical Imaging Platform
Small ( IF 13.3 ) Pub Date : 2022-06-20 , DOI: 10.1002/smll.202202622 Hao Zhu 1 , Zixuan Chen 2 , Yun Chen 1 , Jun-Jie Zhu 2, 3
Small ( IF 13.3 ) Pub Date : 2022-06-20 , DOI: 10.1002/smll.202202622 Hao Zhu 1 , Zixuan Chen 2 , Yun Chen 1 , Jun-Jie Zhu 2, 3
Affiliation
|
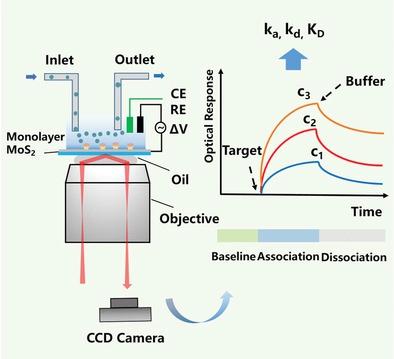
Quantifying the binding kinetics and affinities of protein–small molecule interactions is critical for biomarker validation, drug discovery, and deep understanding of various biological processes at the molecular-scale. Novel approaches are demanded as most common label-free techniques are mass-sensitive, which are not suitable for the detection of small molecule interactions. Here, an optical imaging platform is developed to measure the binding kinetics of both protein–small molecules and protein–ions based on monolayer MoS2, an ultra-thin 2D material whose optical absorption is extremely sensitive to charge. A model is established to calibrate the optical response due to the charged analyte binding and it is applied to quantify the interactions between abl1 kinase and different small-molecule inhibitors. Such a presented method is capable of distinguishing different inhibitors binding to a wild or mutated kinase, which provides guidance for drug evaluation and drug mechanism exploration. The binding kinetics of calcium ions to calmodulin is also measured, further broadening the application field of the method. In addition, the imaging capability allows mapping the local binding kinetics of the molecular interactions with a high resolution, which reveals visible spatial variability and offers a promising tool for studying heterogeneous local interfacial interactions.
中文翻译:

基于单层 MoS2 的光学成像平台对蛋白质-小分子相互作用的亲和力和动力学检测
量化蛋白质-小分子相互作用的结合动力学和亲和力对于生物标志物验证、药物发现和深入了解分子尺度的各种生物过程至关重要。需要新的方法,因为最常见的无标记技术对质量敏感,不适合检测小分子相互作用。在这里,开发了一个光学成像平台,用于测量基于单层 MoS 2的蛋白质-小分子和蛋白质-离子的结合动力学,一种超薄二维材料,其光吸收对电荷极为敏感。建立了一个模型来校准由于带电分析物结合引起的光学响应,并将其用于量化 abl1 激酶和不同小分子抑制剂之间的相互作用。这种提出的方法能够区分与野生或突变激酶结合的不同抑制剂,为药物评估和药物机制探索提供指导。还测量了钙离子与钙调蛋白的结合动力学,进一步拓宽了该方法的应用领域。此外,成像能力允许以高分辨率绘制分子相互作用的局部结合动力学,这揭示了可见的空间变异性,并为研究异质局部界面相互作用提供了一种有前途的工具。
更新日期:2022-06-20
中文翻译:

基于单层 MoS2 的光学成像平台对蛋白质-小分子相互作用的亲和力和动力学检测
量化蛋白质-小分子相互作用的结合动力学和亲和力对于生物标志物验证、药物发现和深入了解分子尺度的各种生物过程至关重要。需要新的方法,因为最常见的无标记技术对质量敏感,不适合检测小分子相互作用。在这里,开发了一个光学成像平台,用于测量基于单层 MoS 2的蛋白质-小分子和蛋白质-离子的结合动力学,一种超薄二维材料,其光吸收对电荷极为敏感。建立了一个模型来校准由于带电分析物结合引起的光学响应,并将其用于量化 abl1 激酶和不同小分子抑制剂之间的相互作用。这种提出的方法能够区分与野生或突变激酶结合的不同抑制剂,为药物评估和药物机制探索提供指导。还测量了钙离子与钙调蛋白的结合动力学,进一步拓宽了该方法的应用领域。此外,成像能力允许以高分辨率绘制分子相互作用的局部结合动力学,这揭示了可见的空间变异性,并为研究异质局部界面相互作用提供了一种有前途的工具。


























 京公网安备 11010802027423号
京公网安备 11010802027423号