当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Undirected, Asymmetric Alkyl Group Functionalizations through Alkane Dehydrogenation
Organic Letters ( IF 5.2 ) Pub Date : 2022-06-20 , DOI: 10.1021/acs.orglett.2c01009 Feng Yu 1, 2 , Renqing Tao 2 , Yiting Su 1, 2 , Guixia Liu 1, 2 , Zheng Huang 1, 2, 3
Organic Letters ( IF 5.2 ) Pub Date : 2022-06-20 , DOI: 10.1021/acs.orglett.2c01009 Feng Yu 1, 2 , Renqing Tao 2 , Yiting Su 1, 2 , Guixia Liu 1, 2 , Zheng Huang 1, 2, 3
Affiliation
|
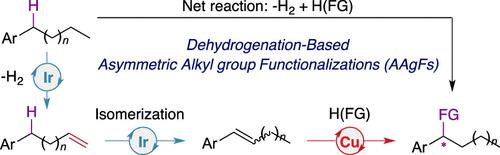
Direct asymmetric alkyl group functionalizations can potentially convert abundant and inexpensive hydrocarbon feedstocks into value-added chiral fine chemicals. Here, we report a one-pot, dehydrogenation-based strategy for enantioselective formal benzylic C(sp3)–H bond borylation. Dehydrogenation of alkylarenes by a pincer-Ir complex produces aryl alkenes via a tandem dehydrogenation/alkene-isomerization catalysis. The subsequent Cu-catalyzed asymmetric alkene hydroboration affords benzylic boronate esters with excellent site- and enantioselectivity. The generality of this strategy has been further demonstrated by asymmetric alkyl group amination.
中文翻译:

通过烷烃脱氢的无向、不对称烷基官能化
直接不对称烷基官能化可以潜在地将丰富且廉价的烃原料转化为增值的手性精细化学品。在这里,我们报告了一种基于脱氢的一锅法,用于对映选择性形式苄基 C(sp 3 )-H 键硼化。通过钳形铱络合物对烷基芳烃的脱氢通过串联脱氢/烯烃异构化催化产生芳基烯烃。随后的 Cu 催化的不对称烯烃硼氢化提供了具有优异位点和对映选择性的苄基硼酸酯。不对称烷基胺化进一步证明了该策略的普遍性。
更新日期:2022-06-20
中文翻译:

通过烷烃脱氢的无向、不对称烷基官能化
直接不对称烷基官能化可以潜在地将丰富且廉价的烃原料转化为增值的手性精细化学品。在这里,我们报告了一种基于脱氢的一锅法,用于对映选择性形式苄基 C(sp 3 )-H 键硼化。通过钳形铱络合物对烷基芳烃的脱氢通过串联脱氢/烯烃异构化催化产生芳基烯烃。随后的 Cu 催化的不对称烯烃硼氢化提供了具有优异位点和对映选择性的苄基硼酸酯。不对称烷基胺化进一步证明了该策略的普遍性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号