当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Asymmetric Cycloadditions of Acyclic Carbonyl Ylides with Aldehydes Catalyzed by a Chiral Binaphthyldiimine-Ni(II) Complex: Enantioselective Synthesis of 1,3-Dioxolanes and Mechanistic Studies by DFT Calculations
Organic Letters ( IF 5.2 ) Pub Date : 2022-06-20 , DOI: 10.1021/acs.orglett.2c01682 Yasunori Toda 1 , Kayo Sato 1 , Kensuke Sato 1 , Kazuma Nagasaki 1 , Hirotaka Nakajima 1 , Ayaka Kikuchi 1 , Kimiya Sukegawa 1 , Hiroyuki Suga 1
Organic Letters ( IF 5.2 ) Pub Date : 2022-06-20 , DOI: 10.1021/acs.orglett.2c01682 Yasunori Toda 1 , Kayo Sato 1 , Kensuke Sato 1 , Kazuma Nagasaki 1 , Hirotaka Nakajima 1 , Ayaka Kikuchi 1 , Kimiya Sukegawa 1 , Hiroyuki Suga 1
Affiliation
|
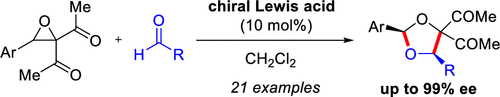
Chiral binaphthyldiimine-Ni(II)-catalyzed asymmetric 1,3-dipolar cycloaddition reactions between acyclic carbonyl ylides generated from donor–acceptor oxiranes and aldehydes are reported. Both aromatic and aliphatic aldehydes could be used as dipolarophiles, providing cis-1,3-dioxolanes with high diastereo- and enantioselectivities. On the basis of mechanistic studies, a monomeric chiral Ni(II) complex was hypothesized to act as the active species for the cycloaddition. The high levels of asymmetric induction are satisfactorily explained by a concerted-asynchronous endo Si-face approach of the aldehyde.
中文翻译:

手性联萘二亚胺-Ni(II) 配合物催化无环羰基叶立德与醛的不对称环加成反应:1,3-二氧戊环的对映选择性合成和通过 DFT 计算进行的机理研究
手性联萘二亚胺-Ni(II) 催化的不对称 1,3-偶极环加成反应在由供体-受体环氧乙烷和醛生成的无环羰基叶立德之间进行。芳香族和脂肪族醛都可以用作亲偶极体,提供具有高非对映选择性和对映选择性的顺式-1,3-二氧戊环。在机理研究的基础上,假设单体手性 Ni(II) 配合物作为环加成的活性物质。高水平的不对称诱导可以通过醛的协同异步endo Si面方法得到令人满意的解释。
更新日期:2022-06-20
中文翻译:

手性联萘二亚胺-Ni(II) 配合物催化无环羰基叶立德与醛的不对称环加成反应:1,3-二氧戊环的对映选择性合成和通过 DFT 计算进行的机理研究
手性联萘二亚胺-Ni(II) 催化的不对称 1,3-偶极环加成反应在由供体-受体环氧乙烷和醛生成的无环羰基叶立德之间进行。芳香族和脂肪族醛都可以用作亲偶极体,提供具有高非对映选择性和对映选择性的顺式-1,3-二氧戊环。在机理研究的基础上,假设单体手性 Ni(II) 配合物作为环加成的活性物质。高水平的不对称诱导可以通过醛的协同异步endo Si面方法得到令人满意的解释。


























 京公网安备 11010802027423号
京公网安备 11010802027423号