Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Label-Free Quantification of Molecular Interaction in Live Red Blood Cells by Tracking Nanometer Scale Membrane Fluctuations
Small ( IF 13.3 ) Pub Date : 2022-06-19 , DOI: 10.1002/smll.202201623 Bo Yao 1, 2 , Yunze Yang 2 , Nanxi Yu 2, 3 , Nongjian Tao 2, 4 , Di Wang 2, 5 , Shaopeng Wang 2, 6 , Fenni Zhang 2, 7
Small ( IF 13.3 ) Pub Date : 2022-06-19 , DOI: 10.1002/smll.202201623 Bo Yao 1, 2 , Yunze Yang 2 , Nanxi Yu 2, 3 , Nongjian Tao 2, 4 , Di Wang 2, 5 , Shaopeng Wang 2, 6 , Fenni Zhang 2, 7
Affiliation
|
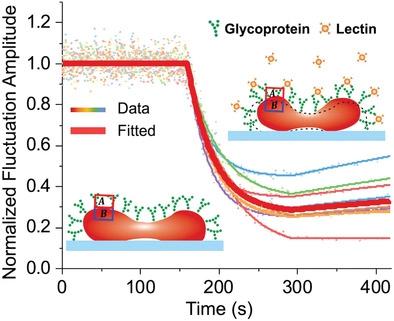
Molecular interactions in live cells play an important role in both cellular functions and drug discovery. Current methods for measuring binding kinetics involve extracting the membrane protein and labeling, while the in situ quantification of molecular interaction with surface plasmon resonance (SPR) imaging mainly worked with fixed cells due to the micro-motion related noises of live cells. Here, an optical imaging method is presented to measure the molecular interaction with live red blood cells by tracking the nanometer membrane fluctuations. The membrane fluctuation dynamics are measured by tracking the membrane displacement during glycoprotein interaction. The data are analyzed with a thermodynamic model to determine the elastic properties of the cell observing reduced membrane fluctuations under fixatives, indicating cell fixations affect membrane mechanical properties. The binding kinetics of glycoprotein to several lectins are obtained by tracking the membrane fluctuation amplitude changes on single live cells. The binding kinetics and strength of different lectins are quite different, indicating the glycoproteins expression heterogeneity in single cells. It is anticipated that the method will contribute to the understanding of mechanisms of cell interaction and communication, and have potential applications in the mechanical assessment of cancer or other diseases at the single-cell level, and screening of membrane protein targeting drugs.
中文翻译:

通过跟踪纳米级膜波动对活红细胞中的分子相互作用进行无标记定量
活细胞中的分子相互作用在细胞功能和药物发现中发挥着重要作用。目前测量结合动力学的方法涉及提取膜蛋白和标记,而由于活细胞的微运动相关噪声,利用表面等离子共振(SPR)成像对分子相互作用进行原位定量主要适用于固定细胞。在这里,提出了一种光学成像方法,通过跟踪纳米膜波动来测量与活红细胞的分子相互作用。通过跟踪糖蛋白相互作用期间的膜位移来测量膜波动动力学。使用热力学模型分析数据,以确定细胞的弹性特性,观察固定剂下膜波动的减少,表明细胞固定会影响膜的机械性能。通过跟踪单个活细胞的膜波动幅度变化获得糖蛋白与几种凝集素的结合动力学。不同凝集素的结合动力学和强度差异很大,表明单细胞中糖蛋白表达的异质性。预计该方法将有助于理解细胞相互作用和通讯的机制,并在单细胞水平上对癌症或其他疾病的机械评估以及膜蛋白靶向药物的筛选方面具有潜在的应用。不同凝集素的结合动力学和强度差异很大,表明单细胞中糖蛋白表达的异质性。预计该方法将有助于理解细胞相互作用和通讯的机制,并在单细胞水平上对癌症或其他疾病的机械评估以及膜蛋白靶向药物的筛选方面具有潜在的应用。不同凝集素的结合动力学和强度差异很大,表明单细胞中糖蛋白表达的异质性。预计该方法将有助于理解细胞相互作用和通讯的机制,并在单细胞水平上对癌症或其他疾病的机械评估以及膜蛋白靶向药物的筛选方面具有潜在的应用。
更新日期:2022-06-19
中文翻译:

通过跟踪纳米级膜波动对活红细胞中的分子相互作用进行无标记定量
活细胞中的分子相互作用在细胞功能和药物发现中发挥着重要作用。目前测量结合动力学的方法涉及提取膜蛋白和标记,而由于活细胞的微运动相关噪声,利用表面等离子共振(SPR)成像对分子相互作用进行原位定量主要适用于固定细胞。在这里,提出了一种光学成像方法,通过跟踪纳米膜波动来测量与活红细胞的分子相互作用。通过跟踪糖蛋白相互作用期间的膜位移来测量膜波动动力学。使用热力学模型分析数据,以确定细胞的弹性特性,观察固定剂下膜波动的减少,表明细胞固定会影响膜的机械性能。通过跟踪单个活细胞的膜波动幅度变化获得糖蛋白与几种凝集素的结合动力学。不同凝集素的结合动力学和强度差异很大,表明单细胞中糖蛋白表达的异质性。预计该方法将有助于理解细胞相互作用和通讯的机制,并在单细胞水平上对癌症或其他疾病的机械评估以及膜蛋白靶向药物的筛选方面具有潜在的应用。不同凝集素的结合动力学和强度差异很大,表明单细胞中糖蛋白表达的异质性。预计该方法将有助于理解细胞相互作用和通讯的机制,并在单细胞水平上对癌症或其他疾病的机械评估以及膜蛋白靶向药物的筛选方面具有潜在的应用。不同凝集素的结合动力学和强度差异很大,表明单细胞中糖蛋白表达的异质性。预计该方法将有助于理解细胞相互作用和通讯的机制,并在单细胞水平上对癌症或其他疾病的机械评估以及膜蛋白靶向药物的筛选方面具有潜在的应用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号