当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Proving Cooperativity of a Catalytic Reaction by Means of Nanoscale Geometry: The Case of Click Reaction
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2022-06-17 , DOI: 10.1021/jacs.2c02556 Cristóbal Quintana 1 , Juan C Ahumada 1 , Guillermo Ahumada 1 , Yaroslav Sobolev 1 , Minju Kim 1, 2 , Atabay Allamyradov 1, 2 , Bartosz A Grzybowski 1, 2, 3
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2022-06-17 , DOI: 10.1021/jacs.2c02556 Cristóbal Quintana 1 , Juan C Ahumada 1 , Guillermo Ahumada 1 , Yaroslav Sobolev 1 , Minju Kim 1, 2 , Atabay Allamyradov 1, 2 , Bartosz A Grzybowski 1, 2, 3
Affiliation
|
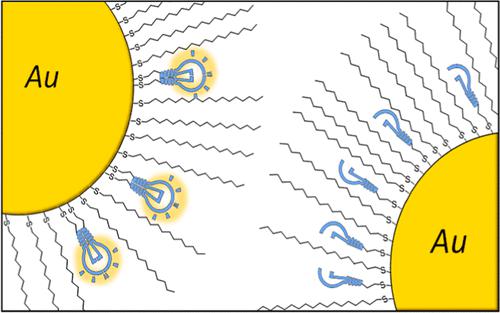
Establishing whether a reaction is catalyzed by a single-metal catalytic center or cooperatively by a fleeting complex encompassing two such centers may be an arduous pursuit requiring detailed kinetic, isotopic, and other types of studies─as illustrated, for instance, by over a decade-long work on single-copper versus di-copper mechanisms of the popular “click" reaction. This paper describes a method to interrogate such cooperative mechanisms by a nanoparticle-based platform in which the probabilities of catalytic units being proximal can be varied systematically and, more importantly, independently of their volume concentration. The method relies on geometrical considerations rather than a detailed knowledge of kinetic equations, yet the scaling trends it yield can distinguish between cooperative and non-cooperative mechanisms.
中文翻译:

用纳米几何尺寸证明催化反应的协同性:点击反应的案例
确定一个反应是由单一金属催化中心催化,还是由包含两个这样的中心的短暂复合物协同催化,可能是一项艰巨的任务,需要详细的动力学、同位素和其他类型的研究——例如,十多年的研究表明- 对流行的“点击”反应的单铜与双铜机制进行长期研究。本文描述了一种通过基于纳米粒子的平台询问这种合作机制的方法,其中催化单元接近的概率可以系统地变化,并且,更重要的是,与它们的体积浓度无关。该方法依赖于几何考虑而不是动力学方程的详细知识,但它产生的缩放趋势可以区分合作机制和非合作机制。
更新日期:2022-06-17
中文翻译:

用纳米几何尺寸证明催化反应的协同性:点击反应的案例
确定一个反应是由单一金属催化中心催化,还是由包含两个这样的中心的短暂复合物协同催化,可能是一项艰巨的任务,需要详细的动力学、同位素和其他类型的研究——例如,十多年的研究表明- 对流行的“点击”反应的单铜与双铜机制进行长期研究。本文描述了一种通过基于纳米粒子的平台询问这种合作机制的方法,其中催化单元接近的概率可以系统地变化,并且,更重要的是,与它们的体积浓度无关。该方法依赖于几何考虑而不是动力学方程的详细知识,但它产生的缩放趋势可以区分合作机制和非合作机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号