Coordination Chemistry Reviews ( IF 20.6 ) Pub Date : 2022-06-16 , DOI: 10.1016/j.ccr.2022.214593 Basudev Maity , Mohd Taher , Shyamalava Mazumdar , Takafumi Ueno
|
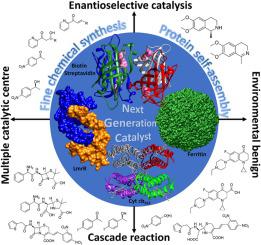
Metalloenzymes play essential roles in biology, whereas artificial metalloenzymes use synthetic metal cofactors for promoting non-natural reactions. In the past decades, tremendous advances have been made in manipulating artificial metalloenzymes for various organic transformation reactions, including C–H activation, C–C coupling, transfer hydrogenation, etc. Advanced methods like “Directed evolution,” “high throughput screening,” and “rational design” have stimulated the artificial metalloenzyme research. Applications of artificial metalloenzymes have been extended to cells for controlling functions like prodrug activation. Usually, for more complicated processes like multistep reactions or isolation of reaction environments, nature uses sophisticated strategies, such as positional assembly and compartmentalization of catalysts. However, artificial metalloenzyme research in this direction is relatively less. Several researchers have designed and constructed various protein assembly structures through metal coordination. However, only a few of them have been tested for catalytic activities. Assembled metalloenzymes have multiple advantages like promoting multistep reactions, stabilizing the catalyst, cooperativity in the reaction, higher-order complexity, sophisticated structures, confinement of reaction, etc. Therefore, systematic investigations on their design, structure, and activity are necessary to represent them as next-generation biocatalysts. In this context, the current review highlights the importance of self-assembled metalloenzymes, available design strategies, current developments, catalytic activities, and the future direction of the research.
中文翻译:

基于蛋白质组装的人工金属酶
金属酶在生物学中发挥着重要作用,而人造金属酶使用合成金属辅因子来促进非天然反应。在过去的几十年中,在操纵人工金属酶进行各种有机转化反应方面取得了巨大进展,包括 C-H 活化、C-C 偶联、转移氢化等。先进的方法,如“定向进化”、“高通量筛选”、和“合理的设计”激发了人工金属酶的研究。人工金属酶的应用已扩展到用于控制前药激活等功能的细胞。通常,对于更复杂的过程,例如多步反应或反应环境的隔离,自然界会使用复杂的策略,例如催化剂的位置组装和分区。然而,人工金属酶这方面的研究相对较少。一些研究人员通过金属配位设计和构建了各种蛋白质组装结构。然而,其中只有少数经过催化活性测试。组装金属酶具有促进多步反应、稳定催化剂、反应协同性、高阶复杂性、复杂结构、反应限制等多种优点。因此,有必要对其设计、结构和活性进行系统研究来代表它们。作为下一代生物催化剂。在此背景下,本综述强调了自组装金属酶的重要性、可用的设计策略、当前的发展、催化活性以及未来的研究方向。



























 京公网安备 11010802027423号
京公网安备 11010802027423号