Chem Catalysis Pub Date : 2022-06-16 , DOI: 10.1016/j.checat.2022.05.018 Min Zhang , Jing Chen , Shijun Huang , Biping Xu , Jin Lin , Weiping Su
|
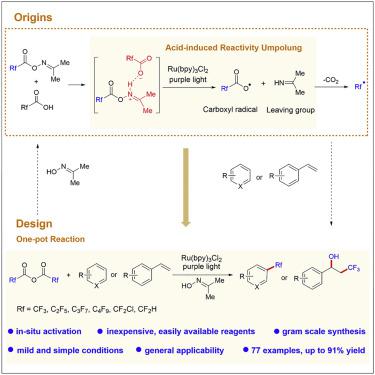
The importance of trifluoromethyl (CF3) group-containing compounds in pharmaceuticals, agrochemicals, and materials has spurred tremendous efforts devoted to the development of methods for incorporation of the trifluoromethyl group into molecular frameworks and discoveries of trifluoromethylating reagents. In this paper, we report a general photocatalytic method for regioselective C–H fluoroalkylation of (hetero)arenes and olefines that uses readily available fluoroalkyl carboxylic anhydrides as fluoroalkylating reagents in the presence of simple acetoxime as an activator. The success achieved in the development of such a photocatalytic C–H fluoroalkylation method is attributed to discovery of the unprecedented acid-triggered reactivity umpolung of acetoxime ester toward single-electron reduction-induced cleavage of N–O σ bond. Such a new reactivity mode of oxime esters will stimulate efforts to expand the synthetic applications of readily available oxime esters.
中文翻译:

乙酰肟酯的酸引发反应性 umpolung 实现(杂)芳烃的光催化氟烷基化
三氟甲基(CF 3) 药物、农用化学品和材料中的含基团化合物激发了巨大的努力,致力于开发将三氟甲基结合到分子框架中的方法和三氟甲基化试剂的发现。在本文中,我们报道了一种用于区域选择性 C-H 氟烷基化(杂)芳烃和烯烃的通用光催化方法,该方法使用容易获得的氟烷基羧酸酐作为氟烷基化试剂,在简单的乙酰肟作为活化剂的情况下。在这种光催化 C-H 氟烷基化方法的开发中取得的成功归因于发现了前所未有的酸引发反应性的乙酰肟酯对单电子还原诱导的 N-O σ 键断裂的反应。


























 京公网安备 11010802027423号
京公网安备 11010802027423号