当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Construction of a Mass-Tagged Oligo Probe Set for Revealing Protein Ratiometric Relationship Associated with EGFR–HER2 Heterodimerization in Living Cells
Analytical Chemistry ( IF 7.4 ) Pub Date : 2022-06-16 , DOI: 10.1021/acs.analchem.1c04989 Xiaoxu Li 1 , Bo Sun 1, 2 , Jianhua Zhu 1 , Moting Qian 1 , Yun Chen 1, 3, 4
Analytical Chemistry ( IF 7.4 ) Pub Date : 2022-06-16 , DOI: 10.1021/acs.analchem.1c04989 Xiaoxu Li 1 , Bo Sun 1, 2 , Jianhua Zhu 1 , Moting Qian 1 , Yun Chen 1, 3, 4
Affiliation
|
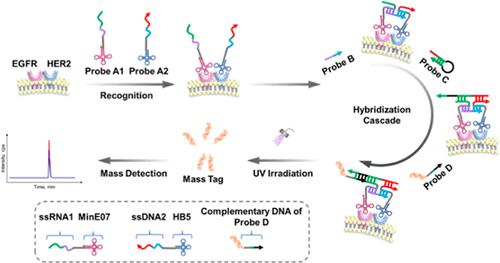
Protein dimerization, as the most common form of protein–protein interaction, can manifest more significant roles in cellular signaling than individual monomers. For example, excessive formation of EGFR–HER2 dimer has been implicated in cancer development and therapeutic resistance in addition to the overexpression of EGFR and HER2 proteins. Thus, quantitative evaluation of these heterodimers in living cells and revelation of their ratiometric relationship with protein monomers in dimerization may provide insights into clinical cancer management. To achieve this goal, the prerequisite is protein heterodimer quantification. Given the current lack of quantitative methods, we constructed a mass-tagged oligo nanoprobe set for quantification of EGFR–HER2 dimer in living cells. The mass-tagged oligo nanoprobe set contained two targeting probes (nucleic acid aptamers), a connector probe, a hairpin probe, and a photocleavable mass-tagged probe. Two distinct aptamers can recognize target protein monomers and initiate the subsequent hybridization cascade involving binding to the connector probe, formation of an initiator strand, opening of a hairpin probe, and ensuing hybridization with a photocleavable mass-tagged probe. Ultimately, the mass tag was released under ultraviolet light and then subjected to mass spectrometric analysis. In this way, the information regarding the interaction between two protein monomers was successfully converted to the quantitative signal of the mass tag. Using the assay, the expression level of EGFR–HER2 dimer and its relationship with individual protein monomers were determined in four breast cancer cell lines. We are among the first to obtain the absolute level of protein heterodimer, and this quantitative information may be vital in understanding the molecular basis of cancer.
中文翻译:

构建用于揭示与活细胞中 EGFR-HER2 异二聚化相关的蛋白质比例关系的质量标记寡核苷酸探针组
蛋白质二聚化作为最常见的蛋白质-蛋白质相互作用形式,在细胞信号传导中的作用比单个单体更重要。例如,除了 EGFR 和 HER2 蛋白的过表达外,EGFR-HER2 二聚体的过度形成与癌症发展和治疗耐药性有关。因此,对活细胞中这些异二聚体的定量评估以及它们与二聚化蛋白质单体的比例关系的揭示可能为临床癌症管理提供见解。为实现这一目标,先决条件是蛋白质异二聚体定量。鉴于目前缺乏定量方法,我们构建了一个质量标记的寡核苷酸纳米探针组,用于定量活细胞中的 EGFR-HER2 二聚体。质量标记寡核苷酸纳米探针组包含两个靶向探针(核酸适体)、一个连接器探针、一个发夹探针和一个光裂解质量标记探针。两种不同的适配体可以识别靶蛋白单体并启动随后的杂交级联,包括与连接探针结合、起始链的形成、发夹探针的打开以及随后与可光裂解的质量标记探针的杂交。最终,质量标签在紫外光下被释放,然后进行质谱分析。通过这种方式,关于两种蛋白质单体之间相互作用的信息成功地转换为质量标签的定量信号。使用化验,在四种乳腺癌细胞系中测定了 EGFR-HER2 二聚体的表达水平及其与单个蛋白质单体的关系。我们是最先获得蛋白质异二聚体绝对水平的人之一,这种定量信息可能对了解癌症的分子基础至关重要。
更新日期:2022-06-16
中文翻译:

构建用于揭示与活细胞中 EGFR-HER2 异二聚化相关的蛋白质比例关系的质量标记寡核苷酸探针组
蛋白质二聚化作为最常见的蛋白质-蛋白质相互作用形式,在细胞信号传导中的作用比单个单体更重要。例如,除了 EGFR 和 HER2 蛋白的过表达外,EGFR-HER2 二聚体的过度形成与癌症发展和治疗耐药性有关。因此,对活细胞中这些异二聚体的定量评估以及它们与二聚化蛋白质单体的比例关系的揭示可能为临床癌症管理提供见解。为实现这一目标,先决条件是蛋白质异二聚体定量。鉴于目前缺乏定量方法,我们构建了一个质量标记的寡核苷酸纳米探针组,用于定量活细胞中的 EGFR-HER2 二聚体。质量标记寡核苷酸纳米探针组包含两个靶向探针(核酸适体)、一个连接器探针、一个发夹探针和一个光裂解质量标记探针。两种不同的适配体可以识别靶蛋白单体并启动随后的杂交级联,包括与连接探针结合、起始链的形成、发夹探针的打开以及随后与可光裂解的质量标记探针的杂交。最终,质量标签在紫外光下被释放,然后进行质谱分析。通过这种方式,关于两种蛋白质单体之间相互作用的信息成功地转换为质量标签的定量信号。使用化验,在四种乳腺癌细胞系中测定了 EGFR-HER2 二聚体的表达水平及其与单个蛋白质单体的关系。我们是最先获得蛋白质异二聚体绝对水平的人之一,这种定量信息可能对了解癌症的分子基础至关重要。


























 京公网安备 11010802027423号
京公网安备 11010802027423号