当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Two Ligands of Interest in Recovering Uranium from the Oceans: The Correct Formation Constants of the Uranyl(VI) Cation with 2,2′-Bipyridyl-6,6′-dicarboxylic Acid and 1,10-Phenanthroline-2,9-dicarboxylic Acid
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2022-06-15 , DOI: 10.1021/acs.inorgchem.2c00775 Erica L Fultz 1 , S Bart Jones 1 , Alexander S Ivanov 2 , Vyacheslav S Bryantsev 2 , Sheng Dai 2 , Robert D Hancock 1
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2022-06-15 , DOI: 10.1021/acs.inorgchem.2c00775 Erica L Fultz 1 , S Bart Jones 1 , Alexander S Ivanov 2 , Vyacheslav S Bryantsev 2 , Sheng Dai 2 , Robert D Hancock 1
Affiliation
|
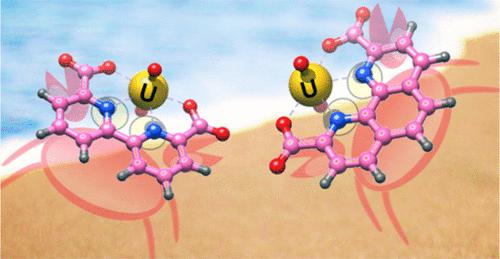
The ligands BDA (2,2′-bipyridyl-6,6′-dicarboxylic acid) and PDA (1,10-phenanthroline-2,9-dicarboxylic acid) are of interest as functional group types for ion-exchange materials for extracting uranium from the oceans, reported in a previous paper for PDA Lashley, M. A. ( Inorg. Chem. 2016 55 10818 10829). Yang, Y. ( Inorg. Chem. 2019, 58, 6064 6074) have published what they claim to be a more accurate result for the formation of the UO22+/PDA complex of log K1 = 22.84 compared with our reported value of log K1 = 16.5, as well as log K1 = 21.52 for the BDA complex. The determination of log K1 for the PDA and BDA complexes with the UO22+ cation was carried out by Yang et al. using a competition reaction between DTPA (diethylenetriamine pentaacetic acid) and BDA or PDA, monitoring the absorbance due to the BDA and PDA ligands. This competition method using absorbance versus pH titrations was developed for determining the formation constants of the complexes of several polypyridyl ligands plus PDA complexes of metal ions, which were too stable for log K determination by competition with protons. A key feature of such titrations is that in the competition reaction, the displacement of the pyridyl donor ligand (e.g., PDA) by the competing ligand (e.g., DTPA), the absorbance spectrum of the displaced pyridyl donor ligand should be observed. Competing ligands used to date have been EDTA (ethylenediamine tetraacetic acid), DTPA, or the hydroxide ion. In the study of Yang et al., no such displaced PDA or BDA was apparent in the absorbance spectra in their titrations so that their reported log K1 values have no validity. Their log K1 values are so much higher than log K1 for the uranyl DTPA complex (∼13.6) that DTPA could not possibly displace BDA or PDA from the uranyl cation, and a competition reaction could not possibly occur. We report the correct value of log K1 = 15.4 (ionic strength = zero) for the uranyl BDA complex, to illustrate the correct determination of such a constant by a competition reaction between BDA and hydroxide, showing how the characteristic absorbance spectrum for a BDA complex, here the UO22+ complex, disappears, and the distinctive absorbance spectrum of the free nonprotonated BDA ligand appears as the pH is increased, and BDA is displaced by the hydroxide ion.
中文翻译:

从海洋中回收铀的两个重要配体:铀酰 (VI) 阳离子与 2,2'-Bipyridyl-6,6'-dicarboxy Acid 和 1,10-Phenanthroline-2,9-dicarboxy Acid 的正确形成常数
配体 BDA(2,2'-联吡啶-6,6'-二羧酸)和 PDA(1,10-菲咯啉-2,9-二羧酸)作为用于提取铀的离子交换材料的官能团类型很有意义来自海洋,在之前的 PDA 论文中报道马萨诸塞州拉什利 (Inorg. Chem. 2016 55 10818 10829)。杨,Y. ( Inorg. Chem. 2019 , 58 , 6064 6074) 发表了他们声称的更准确的结果,与我们报告的 log K 1 值相比,log K 1 = 22.84 的 UO 2 2+ / PDA复合物的形成= 16.5,以及 BDA 复合体的 log K 1 = 21.52。用 UO 2 2+测定PDA 和 BDA 复合物的 log K 1阳离子由杨等人进行。使用 DTPA(二乙烯三胺五乙酸)和 BDA 或 PDA 之间的竞争反应,监测 BDA 和 PDA 配体的吸光度。这种使用吸光度与 pH 滴定的竞争方法被开发用于确定几种多吡啶基配体和金属离子 PDA 配合物的配合物的形成常数,这些配合物对于 log K来说太稳定了通过与质子的竞争来确定。这种滴定的一个关键特征是,在竞争反应中,吡啶基供体配体(例如 PDA)被竞争配体(例如 DTPA)置换,应观察置换的吡啶基供体配体的吸收光谱。迄今为止使用的竞争配体是 EDTA(乙二胺四乙酸)、DTPA 或氢氧根离子。在 Yang 等人的研究中,在他们的滴定吸收光谱中没有明显的这种移位的 PDA 或 BDA,因此他们报告的 log K 1值无效。它们的 log K 1值远高于 log K 1对于铀酰 DTPA 配合物(~13.6),DTPA 不可能从铀酰阳离子中取代 BDA 或 PDA,并且不可能发生竞争反应。我们报告铀酰 BDA 配合物 log K 1 = 15.4(离子强度 = 零)的正确值,以说明通过 BDA 和氢氧化物之间的竞争反应正确确定该常数,显示 BDA 的特征吸收光谱如何配合物,这里是 UO 2 2+配合物,消失了,随着 pH 值的增加,游离的非质子化 BDA 配体的独特吸收光谱出现,并且 BDA 被氢氧根离子取代。
更新日期:2022-06-15
中文翻译:

从海洋中回收铀的两个重要配体:铀酰 (VI) 阳离子与 2,2'-Bipyridyl-6,6'-dicarboxy Acid 和 1,10-Phenanthroline-2,9-dicarboxy Acid 的正确形成常数
配体 BDA(2,2'-联吡啶-6,6'-二羧酸)和 PDA(1,10-菲咯啉-2,9-二羧酸)作为用于提取铀的离子交换材料的官能团类型很有意义来自海洋,在之前的 PDA 论文中报道


























 京公网安备 11010802027423号
京公网安备 11010802027423号