当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Infrared Spectroscopy of Stepwise Hydration Motifs of Sulfur Dioxide
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2022-06-16 , DOI: 10.1021/acs.jpclett.2c01472 Chong Wang 1, 2 , Liangfei Fu 1, 2 , Shuo Yang 1 , Huijun Zheng 1, 2 , Tiantong Wang 1, 2 , Jiao Gao 1 , Mingzhi Su 1, 2 , Jiayue Yang 1 , Guorong Wu 1 , Weiqing Zhang 1 , Zhaojun Zhang 1 , Gang Li 1 , Dong H Zhang 1, 3 , Ling Jiang 1, 3 , Xueming Yang 1, 3, 4
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2022-06-16 , DOI: 10.1021/acs.jpclett.2c01472 Chong Wang 1, 2 , Liangfei Fu 1, 2 , Shuo Yang 1 , Huijun Zheng 1, 2 , Tiantong Wang 1, 2 , Jiao Gao 1 , Mingzhi Su 1, 2 , Jiayue Yang 1 , Guorong Wu 1 , Weiqing Zhang 1 , Zhaojun Zhang 1 , Gang Li 1 , Dong H Zhang 1, 3 , Ling Jiang 1, 3 , Xueming Yang 1, 3, 4
Affiliation
|
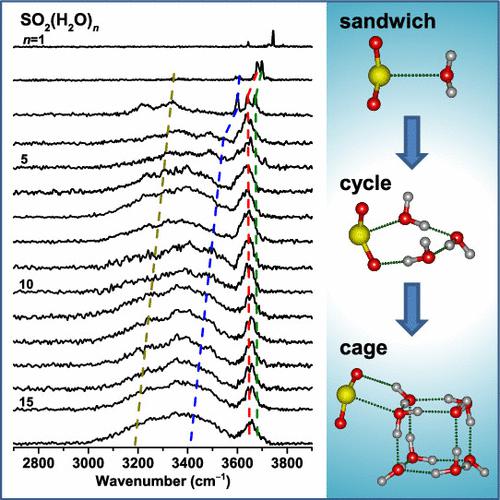
Experimental characterization of microscopic events and behaviors of SO2–H2O interactions is crucial to understanding SO2 atmospheric chemistry but has been proven to be very challenging due to the difficulty in size selection. Here, size-dependent development of SO2 hydrate structure and cluster growth in the SO2(H2O)n (n = 1–16) complexes was probed by infrared spectroscopy based on threshold photoionization using a tunable vacuum ultraviolet free electron laser. Spectral changes with cluster size demonstrate that the sandwich structure initially formed at n = 1 develops into cycle structures with the sulfur and oxygen atoms in a two-dimensional plane (n = 2 and 3) and then into three-dimensional cage structures (n ≥ 4). SO2 is favorably bound to the surface of larger water clusters. These stepwise features of SO2 hydration on various sized water clusters contribute to understanding the reactive sites and electrophilicity of SO2 on cloud droplets, which may have important atmospheric implications for studying the SO2-containing aerosol systems.
中文翻译:

二氧化硫逐步水合基序的红外光谱
SO 2 -H 2 O 相互作用的微观事件和行为的实验表征对于理解 SO 2大气化学至关重要,但由于尺寸选择的困难已被证明非常具有挑战性。在这里,使用可调谐真空紫外自由电子激光器基于阈值光电离的红外光谱法探测了 SO 2 (H 2 O) n ( n = 1-16) 配合物中 SO 2水合物结构的尺寸依赖性发展和簇生长。光谱随簇大小的变化表明三明治结构最初形成于n= 1 发展成具有硫和氧原子在二维平面中的循环结构(n = 2 和 3),然后发展成三维笼状结构(n ≥ 4)。SO 2有利地结合到较大水簇的表面。SO 2在各种大小的水簇上水合的逐步特征有助于了解 SO 2在云滴上的反应位点和亲电性,这可能对研究含 SO 2的气溶胶系统具有重要的大气影响。
更新日期:2022-06-16
中文翻译:

二氧化硫逐步水合基序的红外光谱
SO 2 -H 2 O 相互作用的微观事件和行为的实验表征对于理解 SO 2大气化学至关重要,但由于尺寸选择的困难已被证明非常具有挑战性。在这里,使用可调谐真空紫外自由电子激光器基于阈值光电离的红外光谱法探测了 SO 2 (H 2 O) n ( n = 1-16) 配合物中 SO 2水合物结构的尺寸依赖性发展和簇生长。光谱随簇大小的变化表明三明治结构最初形成于n= 1 发展成具有硫和氧原子在二维平面中的循环结构(n = 2 和 3),然后发展成三维笼状结构(n ≥ 4)。SO 2有利地结合到较大水簇的表面。SO 2在各种大小的水簇上水合的逐步特征有助于了解 SO 2在云滴上的反应位点和亲电性,这可能对研究含 SO 2的气溶胶系统具有重要的大气影响。


























 京公网安备 11010802027423号
京公网安备 11010802027423号