Journal of Hepatology ( IF 25.7 ) Pub Date : 2022-06-15 , DOI: 10.1016/j.jhep.2022.05.031 Man-Fung Yuen 1 , Jeong Heo 2 , Hiromitsu Kumada 3 , Fumitaka Suzuki 3 , Yoshiyuki Suzuki 4 , Qing Xie 5 , Jidong Jia 6 , Yoshiyasu Karino 7 , Jinlin Hou 8 , Kazuaki Chayama 9 , Michio Imamura 10 , Judy Y Lao-Tan 11 , Seng Gee Lim 12 , Yasuhito Tanaka 13 , Wen Xie 14 , Jung-Hwan Yoon 15 , Zhongping Duan 16 , Masayuki Kurosaki 17 , Sung-Jae Park 18 , Madalinee Eternity Labio 19 , Rajneesh Kumar 20 , Young-Oh Kweon 21 , Hyung Joon Yim 22 , Yu Tao 23 , Jennifer Cremer 24 , Robert Elston 25 , Matt Davies 26 , Sharon Baptiste-Brown 27 , Kelong Han 28 , Fiona M Campbell 25 , Melanie Paff 29 , Dickens Theodore 24
|
Background & Aims
Bepirovirsen, an antisense oligonucleotide targeting pregenomic and mRNA transcripts of HBV, has been conjugated to N-acetyl galactosamine (GSK3389404) to enhance hepatocyte delivery. This dose-finding study was the first to assess GSK3389404 for chronic HBV infection.
Methods
This phase IIa, randomised, double-blind, placebo-controlled, 2-part study was conducted in 22 centres in Asia (NCT03020745). Pharmacokinetic findings from Part 1 informed Part 2 dosing. In Part 2, patients with chronic hepatitis B on nucleos(t)ide analogue therapy were randomised 11:2 to GSK3389404 (30, 60, 120 mg weekly or 120 mg bi-weekly) or placebo until Day 85. Coprimary endpoints included HBsAg response (≥1.5 log10 IU/ml reduction from baseline) rate, safety and pharmacokinetics.
Results
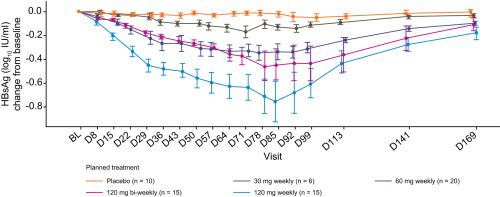
Parts 1 and 2 included 12 (9 GSK3389404, 3 placebo) and 66 patients (56 GSK3389404, 10 placebo), respectively. In Part 2, one patient each in the 60 mg weekly, 120 mg weekly and 120 mg bi-weekly arms achieved a HBsAg response. HBsAg reductions were dose-dependent (Day 85: mean 0.34 [60 mg weekly] to 0.75 log10 IU/ml [120 mg weekly]) and occurred in hepatitis B e antigen-positive and -negative patients. No patient achieved HBsAg seroclearance. 43/56 (77%) GSK3389404- and 9/10 (90%) placebo-treated patients reported adverse events. No deaths were reported. Alanine aminotransferase flares (>2x upper limit of normal) occurred in 2 GSK3389404-treated patients (120 mg weekly, 120 mg bi-weekly); both were associated with decreased HBsAg, but neither was considered a responder. GSK3389404 plasma concentrations peaked 2–4 hours post dose; mean plasma half-life was 3–5 hours.
Conclusions
GSK3389404 showed an acceptable safety profile and target engagement, with dose-dependent reductions in HBsAg. However, no efficacious dosing regimen was identified.
Clinical trial number
NCT03020745.
Lay summary
Hepatitis B virus (HBV) can result in chronic HBV infection, which may ultimately lead to chronic liver disease, primary liver cancer and death; HBV proteins may prevent the immune system from successfully controlling the virus. GSK3389404 is an investigational agent that targets HBV RNA, resulting in reduced viral protein production. This study assessed the safety of GSK3389404 and its ability to reduce the viral proteins in patients with chronic HBV infection. GSK3389404 showed dose-dependent reduction in hepatitis B surface antigen, with an acceptable safety profile. While no clear optimal dose was identified, the findings from this study may help in the development of improved treatment options for patients with chronic HBV infections.
中文翻译:

GSK3389404 在接受稳定核苷(酸)治疗的慢性乙型肝炎患者中的 IIa 期、随机、双盲研究
背景与目标
Bepirovirsen 是一种靶向 HBV 前基因组和 mRNA 转录物的反义寡核苷酸,已与N-乙酰半乳糖胺 (GSK3389404)结合以增强肝细胞递送。该剂量探索研究首次评估 GSK3389404 对慢性 HBV 感染的影响。
方法
这项 IIa 期随机、双盲、安慰剂对照、分为两部分的研究在亚洲的 22 个中心进行 (NCT03020745)。第 1 部分的药代动力学结果为第 2 部分的给药提供了依据。在第 2 部分中,接受核苷(酸)类似物治疗的慢性乙型肝炎患者按 11:2 的比例随机分配至 GSK3389404(每周 30、60、120 毫克或每两周 120 毫克)或安慰剂,直至第 85 天。共同主要终点包括 HBsAg 反应(≥1.5 log 10 IU/ml 从基线减少)率、安全性和药代动力学。
结果
第 1 部分和第 2 部分分别包括 12 名患者(9 名 GSK3389404,3 名安慰剂)和 66 名患者(56 名 GSK3389404,10 名安慰剂)。在第 2 部分中,每周 60 毫克、每周 120 毫克和每两周 120 毫克的组中各有一名患者获得了 HBsAg 反应。HBsAg 降低呈剂量依赖性(第 85 天:平均 0.34 [每周 60 mg] 至 0.75 log 10IU/ml [每周 120 毫克]) 并发生在乙型肝炎 e 抗原阳性和阴性患者中。没有患者达到 HBsAg 血清学清除。43/56 (77%) GSK3389404 和 9/10 (90%) 接受安慰剂治疗的患者报告了不良事件。没有死亡报告。2 名接受 GSK3389404 治疗的患者(每周 120 毫克,每两周 120 毫克)发生丙氨酸转氨酶突然升高(> 正常上限的 2 倍);两者均与 HBsAg 降低有关,但均未被视为应答者。GSK3389404 血浆浓度在给药后 2-4 小时达到峰值;平均血浆半衰期为 3-5 小时。
结论
GSK3389404 显示出可接受的安全性和目标参与度,HBsAg 剂量依赖性降低。然而,没有确定有效的给药方案。
临床试验编号
NCT03020745。
外行总结
乙型肝炎病毒(HBV)可导致慢性HBV感染,最终可能导致慢性肝病、原发性肝癌和死亡;HBV 蛋白可能会阻止免疫系统成功控制病毒。GSK3389404 是一种靶向 HBV RNA 的研究药物,可减少病毒蛋白的产生。本研究评估了 GSK3389404 的安全性及其降低慢性 HBV 感染患者病毒蛋白的能力。GSK3389404 显示出乙型肝炎表面抗原的剂量依赖性降低,具有可接受的安全性。虽然没有确定明确的最佳剂量,但这项研究的结果可能有助于为慢性 HBV 感染患者开发改进的治疗方案。


























 京公网安备 11010802027423号
京公网安备 11010802027423号