当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Assessing (Mo2/3Sc1/3)2C and (Mo2/3Sc1/3)2CT2 (T = −O, −OH, and −F) i-MXenes as High-Performance Electrode Materials for Lithium and Non-Lithium Ion Batteries
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2022-06-15 , DOI: 10.1021/acs.jpcc.2c02280 Tianyu Bai 1 , Haoliang Liu 1 , Baiyi Chen 1 , Yiding Qiu 1 , Haojie Dong 1 , Kai Wu 1 , Yonghong Cheng 1 , Bing Xiao 1
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2022-06-15 , DOI: 10.1021/acs.jpcc.2c02280 Tianyu Bai 1 , Haoliang Liu 1 , Baiyi Chen 1 , Yiding Qiu 1 , Haojie Dong 1 , Kai Wu 1 , Yonghong Cheng 1 , Bing Xiao 1
Affiliation
|
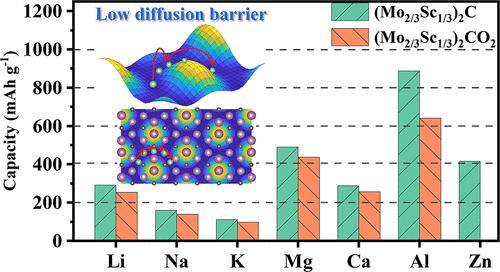
Employing first-principles calculations, the energy storage properties and ion diffusion dynamics of Li+, Na+, K+, Mg2+, Ca2+, Zn2+, and Al3+ on bare (Mo2/3Sc1/3)2C and surface-functionalized (Mo2/3Sc1/3)2CT2 (T = −O, −OH, and −F) i-MXenes are predicted. The investigated i-MXenes show weak adsorption ability to the Zn2+ ion regardless of the surface terminations, excluding their use as anodes for Zn ion batteries. The first-principles molecular dynamics simulations indicate that the adsorption of alkaline (earth) metal ions and Al3+ on (Mo2/3Sc1/3)2C(OH)2 and (Mo2/3Sc1/3)2CF2 causes the surface reaction between metal ions and surface terminations, leading to the formation of metal hydride or fluorite overlayers covering the underneath remaining i-MXenes. We find that the bare (Mo2/3Sc1/3)2C and (Mo2/3Sc1/3)2CO2 are suitable candidates for use as anodes for alkaline (earth) metal and Al ion batteries. Specifically, both (Mo2/3Sc1/3)2C and (Mo2/3Sc1/3)2CO2 i-MXenes show good ion storage capacities, ideal open circuit voltages, and fast ion diffusion dynamics for alkaline (earth) metal ions. Notably, the predicted theoretical capacities of (Mo2/3Sc1/3)2C ((Mo2/3Sc1/3)2CO2) for Li+, Mg2+, and Al3+ are 291 mAh g–1 (254 mAh g–1), 490 mAh g–1 (436 mAh g–1), and 886 mAh g–1 (640 mAh g–1), respectively. In addition, the calculated open circuit voltage profiles of Li+, Mg2+, and Al3+ exhibit the small on-set voltage and the board plateau region. The climbing image-nudged elastic band predicts that the diffusion energies of Li+, Na+, K+, and Ca2+ ions on bare (Mo2/3Sc1/3)2C are extremely small (<0.05 eV), and the O-terminated surface show higher diffusion energies for various metal ions. Overall, (Mo2/3Sc1/3)2C and (Mo2/3Sc1/3)2CO2 are good electrode materials for fast charging alkaline (earth) metal ion batteries and supercapacitors.
中文翻译:

评估 (Mo2/3Sc1/3)2C 和 (Mo2/3Sc1/3)2CT2 (T = -O、-OH 和 -F) i-MXenes 作为锂和非锂离子电池的高性能电极材料
采用第一性原理计算,Li +、Na +、K +、Mg 2+、Ca 2+、Zn 2+和Al 3+在裸(Mo 2/3 Sc 1/ 3 ) 2 C 和表面功能化 (Mo 2/3 Sc 1/3 ) 2 CT 2 (T = -O、-OH 和 -F) i-MXenes 被预测。所研究的 i-MXenes 对 Zn 2+的吸附能力较弱离子,无论表面终端如何,不包括它们用作锌离子电池的阳极。第一性原理分子动力学模拟表明碱(土)金属离子和Al 3+在(Mo 2/3 Sc 1/3 ) 2 C(OH) 2和(Mo 2/3 Sc 1/3 )上的吸附2 CF 2引起金属离子和表面末端之间的表面反应,导致形成金属氢化物或萤石覆盖层,覆盖下面剩余的 i-MXenes。我们发现裸露的 (Mo 2/3 Sc 1/3 ) 2 C 和 (Mo 2/3 Sc 1/3) 2 CO 2适合用作碱(土)金属和铝离子电池的阳极。具体而言,(Mo 2/3 Sc 1/3 ) 2 C 和 (Mo 2/3 Sc 1/3 ) 2 CO 2 i-MXenes 均显示出良好的离子存储能力、理想的开路电压和快速的碱性离子扩散动力学(土)金属离子。值得注意的是,(Mo 2/3 Sc 1/3 ) 2 C ((Mo 2/3 Sc 1/3 ) 2 CO 2 )对Li + , Mg 2+的预测理论容量, 和 Al 3+分别为 291 mAh g –1 (254 mAh g –1 )、490 mAh g –1 (436 mAh g –1 ) 和 886 mAh g –1 (640 mAh g –1 )。此外,计算的Li +、Mg 2+和Al 3+的开路电压分布表现出小的启动电压和电路板平台区域。爬升图像微调弹性带预测 Li +、Na +、K +和 Ca 2+离子在裸 (Mo 2/3 Sc 1/3 ) 2上的扩散能C 极小(<0.05 eV),O 端面对各种金属离子表现出更高的扩散能。总体而言,(Mo 2/3 Sc 1/3 ) 2 C 和(Mo 2/3 Sc 1/3 ) 2 CO 2是用于快速充电碱(土)金属离子电池和超级电容器的良好电极材料。
更新日期:2022-06-15
中文翻译:

评估 (Mo2/3Sc1/3)2C 和 (Mo2/3Sc1/3)2CT2 (T = -O、-OH 和 -F) i-MXenes 作为锂和非锂离子电池的高性能电极材料
采用第一性原理计算,Li +、Na +、K +、Mg 2+、Ca 2+、Zn 2+和Al 3+在裸(Mo 2/3 Sc 1/ 3 ) 2 C 和表面功能化 (Mo 2/3 Sc 1/3 ) 2 CT 2 (T = -O、-OH 和 -F) i-MXenes 被预测。所研究的 i-MXenes 对 Zn 2+的吸附能力较弱离子,无论表面终端如何,不包括它们用作锌离子电池的阳极。第一性原理分子动力学模拟表明碱(土)金属离子和Al 3+在(Mo 2/3 Sc 1/3 ) 2 C(OH) 2和(Mo 2/3 Sc 1/3 )上的吸附2 CF 2引起金属离子和表面末端之间的表面反应,导致形成金属氢化物或萤石覆盖层,覆盖下面剩余的 i-MXenes。我们发现裸露的 (Mo 2/3 Sc 1/3 ) 2 C 和 (Mo 2/3 Sc 1/3) 2 CO 2适合用作碱(土)金属和铝离子电池的阳极。具体而言,(Mo 2/3 Sc 1/3 ) 2 C 和 (Mo 2/3 Sc 1/3 ) 2 CO 2 i-MXenes 均显示出良好的离子存储能力、理想的开路电压和快速的碱性离子扩散动力学(土)金属离子。值得注意的是,(Mo 2/3 Sc 1/3 ) 2 C ((Mo 2/3 Sc 1/3 ) 2 CO 2 )对Li + , Mg 2+的预测理论容量, 和 Al 3+分别为 291 mAh g –1 (254 mAh g –1 )、490 mAh g –1 (436 mAh g –1 ) 和 886 mAh g –1 (640 mAh g –1 )。此外,计算的Li +、Mg 2+和Al 3+的开路电压分布表现出小的启动电压和电路板平台区域。爬升图像微调弹性带预测 Li +、Na +、K +和 Ca 2+离子在裸 (Mo 2/3 Sc 1/3 ) 2上的扩散能C 极小(<0.05 eV),O 端面对各种金属离子表现出更高的扩散能。总体而言,(Mo 2/3 Sc 1/3 ) 2 C 和(Mo 2/3 Sc 1/3 ) 2 CO 2是用于快速充电碱(土)金属离子电池和超级电容器的良好电极材料。



























 京公网安备 11010802027423号
京公网安备 11010802027423号